The Laboklin expert « Round Tables » have become a well-known event, whereby invited experts engage in a moderated in-depth discussion with questions from the audience. We have compiled some interesting aspects of the round-table on immune haemolytic anaemia (IMHA) for you here.
Among the participants who attended our expert round table on IMHA were: Prof Dr Barbara Kohn, from the Freie Universität Berlin, haematology is one of her main areas of research; Dr Esther Haßdenteufel, from Justus Liebig University Giessen, an intensive care physician, who frequently treats patients with IMHA; Dr Barbara Glanemann, from the Royal Veterinary College England, who played a key role in drawing up the ACVIM Consensus Statement on IMHA; Dr Annemarie Baur-Kaufhold, head of the haematology department at Laboklin; Prof Dr Wolfgang Bäumer, Director of the Institute of Pharmacology and Toxicology in the Department of Veterinary Medicine at the Freie Universität of Berlin.
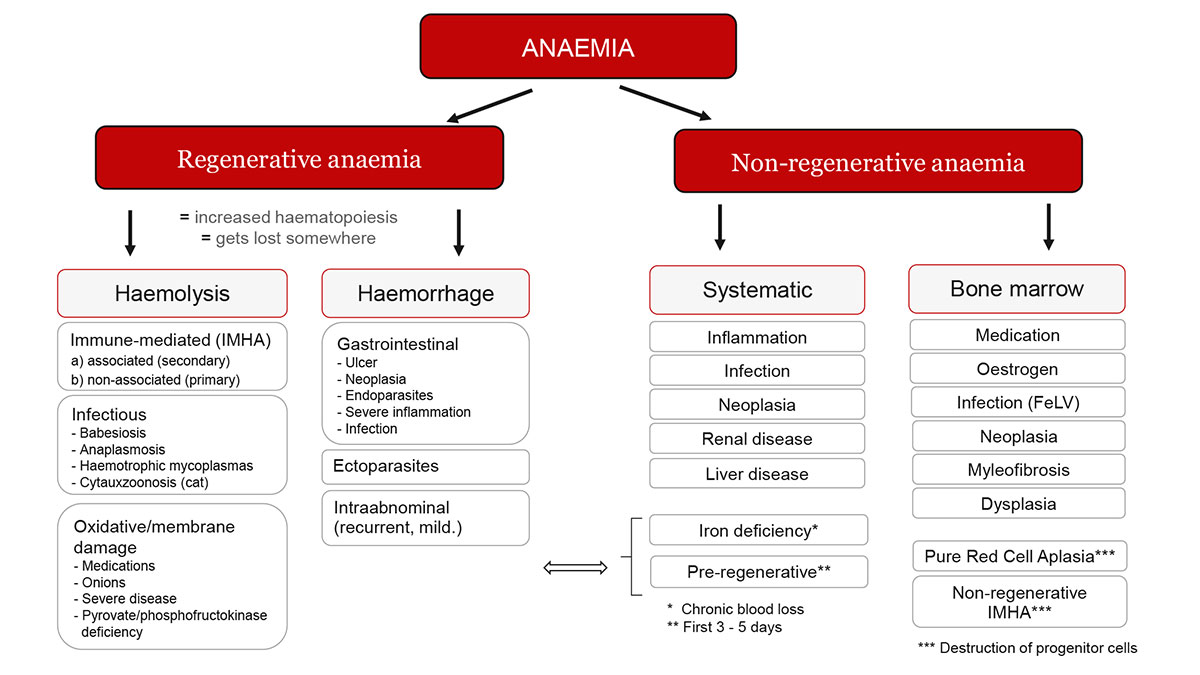
To introduce the topic of anaemia, Dr Glanemann gave an overview of the different forms of anaemias dividing them into regenerative and non-regenerative anaemias. Regenerative anaemia is when reticulocytes are present. Blood loss or haemolysis are possible causes.
Haemolysis can be caused by various mechanisms. The most well-known form of haemolysis is immune-mediated haemolytic anaemia (IMHA), in where the body’s own immune system attacks the erythrocytes. However, there are also some infectious agents that destroy erythrocytes.
Membrane damage to the erythrocytes can also cause them to lyse. This has been described in oxidative processes (e.g. onion or zinc toxicity) or congenital diseases (e.g. pyruvate kinase deficiency).
Non-regenerative anaemias are usually caused by diseases of the organ systems (including endocrinopathies), inflammation or a problem with the bone marrow.
Anaemias that occur acutely (within 3 – 5 days) may not yet be regenerative because the bone marrow needs time to react (pre-regenerative). This also applies to acute haemolysis.
-
Fig. 1: Differential diagnoses of anaemia
Image source: Dr Jennifer von Luckner
-
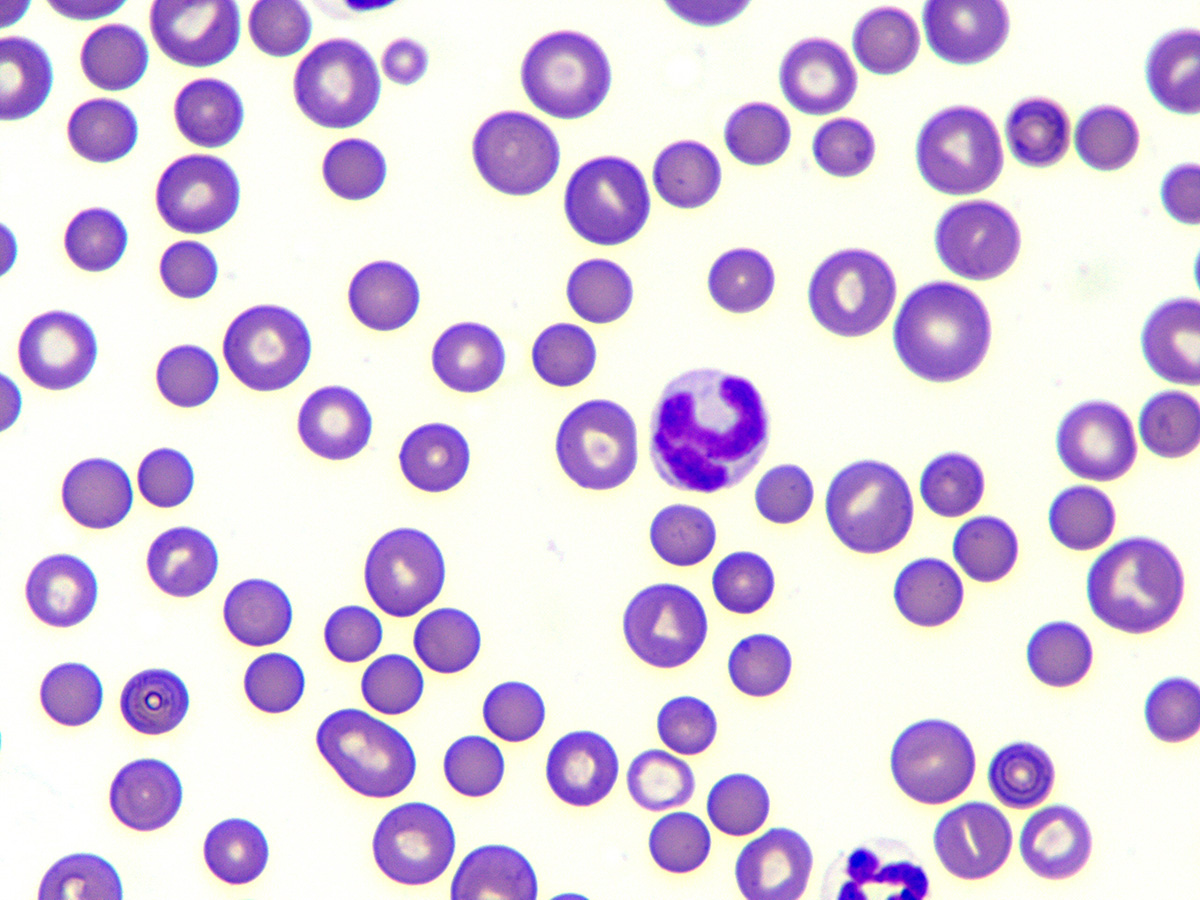
Fig. 2: Spherocytes
Image source: Laboklin
-
Fig. 3: Autoagglutination
Image source: Dr Jennifer von Luckner
There are also IMHA forms in which precursor cells of the erythroid series are attacked. In these cases, the signs of regeneration typical of haemolysis are absent in the blood.
Dr Baur-Kaufhold added that in these non-regenerative IMHA forms, a distinction is made between « pure red cell aplasia » (PRCA) and precursor targeted IMHA (= PIMA, also known as non-regenerative IMHA = nrIMHA) on the basis of the bone marrow findings. In PRCA, erythroid hypo-/aplasia is found in the bone marrow, whereas in PIMA/nrIMHA, erythroid hyperplasia is present. Prof Kohn added that PRCA is seen more frequently in cats than in dogs.
Dr Baur-Kaufhold addressed the question of the difference between intravascular and extravascular haemolysis and explained that in intravascular haemolysis, the erythrocytes are destroyed directly in the bloodstream. The haemoglobin released into the bloodstream is visible by the red colour of the serum and urine. In contrast, by extravascular haemolysis the erythrocytes are destroyed in the liver, spleen and/or bone marrow.
Dr Glanemann added that intravascular IMHA is often more severe and has a worse prognosis than extravascular IMHA.
Prof Kohn reported that new erythrocyte formation and destruction can balance each other out. Such protracted, chronic forms are more difficult to diagnose. Here it is particularly important that other causes of anaemia (such as hidden bleeding) are excluded. The Coombs test can furthermore be helpful.
It is interesting to note at this point that protracted IMHA is more frequently seen in cats.
In some patients with IMHA, thrombocytopenia is present at the same time as anaemia.
Dr Glanemann explained that we refer to Evans syndrome when both the thrombocytopenia and the anaemia occur due to immune-mediated cell destruction. However, platelets are often also consumed as part of disseminated intravascular coagulation (DIC) that accompanies IMHA. The distinction is not always easy. As a rule of thumb, immune-mediated thrombocytopenia (IMTP) usually results in very severe thrombocytopenia, whereas DIC is more moderate. As IMTP rarely occurs without detectable antiplatelet antibodies, their determination can be indicative.
Prof Kohn explained that IMHA can be primary (another term: non-associated) or secondary (another term: associated). To stick with the old terms, primary means that no possible trigger for IMHA could be found. Secondary means that a possible underlying (associated) disease or trigger has been identified. In principle, any disease and many external triggers can cause IMHA. A very careful examination, including imaging, is always necessary.
With regard to vaccination, Dr Glanemann pointed out that there is still no concrete evidence that vaccination triggers IMHA. In relation to the high number of vaccinations, only very few cases of IMHA can be time-linked to vaccinations. Prof Kohn interjected that this is certainly the case statistically. However, in some cases, IMHA occurs in direct temporal connection with the vaccination, and therefore the vaccination may possibly be a trigger.
This raises the question of how to deal with further vaccinations in such patients. Prof Kohn advised adhering closely to the recommendations of the Standing Committee on Vaccination in Veterinary Medicine (StIKo Vet) and only carrying out core vaccinations after an individual risk assessment.
It performs out titer checks for parvo virus and distemper and only vaccinates patients if there is no Antibody titre. Unfortunately, the titre control is unreliable for checking vaccination protection against leptospirosis, so annual revaccination is recommended.
Dr Baur-Kaufhold gave an overview of diagnostics. Firstly, attention should be paid for evidence of possible non-immune mediated processes (e.g. onion, zinc, stay abroad). The breed can also be interesting. For example, pyruvate kinase deficiency has been described in Somali and Abyssinian cats, very rarely in other cat breeds such as Maine Coon and also in some dog breeds. Although congenital, it can only become clinically apparent later in life.
On the other hand, there are certain dog breeds such as Cocker Spaniels that appear to develop IMHA more frequently. A blood smear can identify oxidative damage to erythrocytes (e.g. Heinz bodies).
In any case, infectious agents should be excluded. Prof Kohn therefore routinely checks the following pathogens in IMHA patients:
- Dogs are always tested for Anaplasma and Babesia spp. (using PCR – serology only indicates contact and can also be negative in the case of very early infection). In dogs that are living in endemic regions or that have travelled to or were imported from such regions a full profile for vector borne diseases should be performed (antibodies against Ehrlichia canis and Leishmania infantum, Dirofilaria immitis antigen and filarias PCR or Knott test, Hepatozoon spp. PCR).
- Cats are tested for haemotropic mycoplasmas and piroplasmas (including Babesia and Cytauxzoon spp.) by PCR, FeLV (always including provirus PCR) and FIV.
Typical signs of IMHA, as explained by Dr Baur-Kaufhold, are spherocytes (Fig. 2) in the blood smear (dog), presence of autoagglutination and a positive Coombs test. If a non-regenerative form of IMHA is suspected, a bone marrow examination should always be performed after excluding other possible causes of non-regenerative anaemia.
She further reported that spherocytes are formed after partial phagocytosis by macrophages. Spherocytes are smaller than normal erythrocytes and have no central palor. It is generally stated that a proportion of 5 spherocytes per high power field (hpf) is considered suspicious. The more spherocytes there are, the more likely IMHA is.
Single spherocytes, on the other hand, should not be over-interpreted, as other diseases can also lead to their formation. In cats, spherocytes are not normally used for diagnosis, as this species naturally has relatively small erythrocytes with little to no central palor. In many cases, autoagglutination can be visible in the tube as clumpy appearing blood (Fig. 3). If the agglutinated erythrocytes are not already evident, a slide test should be performed: For this purpose, 1 drop of blood is mixed with 1 – 2 drops of saline and examined for fine clumps or – under the microscope – for erythrocytes sticking together. It is correct to wash the erythrocytes before checking for autoagglutination, because non-specific reactions can lead to an appearance similar to agglutination.
Prof Kohn stated that the Coombs test is used to test immune reactions against the body’s own erythrocytes. For this purpose, the blood sample is analysed for the agglutination of erythrocytes after the addition of antibody-containing test sera.
On a scientific basis, a distinction can be made between the different antibodies involved. In everyday practice, so-called polyvalent tests are sufficient for the diagnosis of IMHA. A positive result indicates an immunological event. Dr Glanemann points out that false-negative results can occur. A negative Coombs test therefore does not rule out IMHA. Dr Baur-Kaufhold reports that the Coombs test cannot always be evaluated. If, for example, autoagglutination of the erythrocytes is present, the Coombs test based on agglutination cannot be read. Other reasons are the submission of incorrect material and the destruction of the red cells in an overaged blood sample, because of inadequate storage, or during transport.
When asked whether the bilirubin concentration always rises in IMHA, Dr Glanemann answers: Bilirubin is released from destroyed erythrocytes. Hyperbilirubinaemia only becomes visible when the liver is unable to cope with its breakdown. It is almost always seen with intravascular haemolysis. But this is not always the case with extravascular haemolysis (especially in protracted forms). The severity of the hyperbilirubinaemia is prognostically relevant. In cats, IMHA often progresses without hyperbilirubinaemia, especially in nrIMHA.
The next section explored the various treatment options. Dr Haßdenteufel explained that a haematocrit guideline value of 12 % is often given when it comes to the requirement of a blood transfusion. However, this value is primarily intended as a guideline. If the anaemia has developed very quickly (usually pre-regenerative), the patient has had little time to get used to the low oxygen transport and as such, the transfusion may also have to be carried out at a higher haematocrit value; conversely the patients that have been anaemic for a longer period may even tolerate a lower haematocrit. In either case, it is always important to examine the individual patient for signs of acute hypoxia such as tachycardia and polypnoea. If these are present, a blood transfusion is indicated.
On the subject of xenotransfusion in cats (transfusion of dog blood), Dr Haßdenteufel reports that this is done in emergency situations when no suitable donor can be found. Cats cope well with it and it can be a real lifesaver. However, it is important to note: Canine erythrocytes are destroyed just a few days after transfusion, therefore they only help in the short term. In addition, the cat must never receive another dog blood transfusion as antibodies are formed very quickly, which lead to a fulminant transfusion reaction if such transfusion is repeated. It is essential that owners are also made aware of this!
Immunosuppressive therapy is usually initiated with prednisolone. Prof Bäumer agreed with the other experts that this is an adequate standard. In severe forms, particularly cases with intravascular haemolysis, severe hyperbilirubinaemia or severe autoagglutination, the experts like to start a second immunosuppressant early on. They also concurred that if the haematocrit continues to fall within 48 hours of starting the treatment and/ or the patient requires multiple transfusions, a second immunosuppressant should be used. This is also recommended if the patient is expected to show severe side effects to the prednisolone and a faster reduction of the glucocorticoid is targeted in the long term. This is often the case in large dogs with a body weight over 25 kg or cat breeds with a predisposition to diabetes mellitus. A third immunosuppressant, on the other hand, is not often indicated as this leads to an increased risk of side effects (including septicaemia) with an unclear benefit.
Some participants questioned whether prednisolone can be used before the results for the infectious agents are available. Prof Kohn advised starting immunosuppressive prednisolone therapy if there is any doubt. If there is a strong suspicion of an Anaplasma spp. or Babesia spp. infection, for example, doxycycline or imidocarb may need to be administered.
The use of immunoglobulins is often discussed for treatment failures. Dr Glanemann emphasised that there is no evidence for their effectiveness in IMHA. However, if no other measure is successful, immunoglobulins can be tried before or after a splenectomy – which is also considered the « ultima ratio » in IMHA.
Parallel to immunosuppressive therapy, the ACVIM Consensus Statement recommends treatment with anticoagulants. Dr Haßdenteufel corroborated that patients with IMHA tend to form thrombi, which can lead to perfusion disorders. This is also the case when thrombocytopenia is present. The ACVIM Consensus Statement specifies a lower limit of 30,000 platelets/μl for antithrombotic therapy. Heparin derivatives (unfractionated and low-molecular-weight heparin) are prioritised over clopidogrel. However, heparin treatment should be monitored by regular coagulation checks or with the help of a factor Xa determination. Dr Glanemann pointed out that this is unrealistic under practical conditions. She herself has had good experiences with clopidogrel. The advantage of clopidogrel is that it can be administered orally. This is because, according to the ACVIM Consensus Statement, the treatment should be administered for the entire duration of prednisolone treatment.
Finally, the duration and monitoring of the therapy came into focus. Prof Kohn reiterated that a treatment period of several months should be adhered to. In the event of recurrences, lifelong treatment may be necessary. This is often the case with cats in particular. Dr Glanemann monitors the therapy based on the haematocrit, preferably in conjunction with a determination of the reticulocyte count. If the reticulocytes fall while the haematocrit remains stable or rises, this is a good sign. After the patient has recovered from the disease and all medication has been discontinued, it is advisable to check the haematological values for a further 4 weeks to confirm that the patient is really in remission. After that, no further blood tests are necessary. The relapse rate for dogs with primary/ non-associated IMHA is around 15 – 20 %.
Dr Jennifer von Luckner