The new version of the German Veterinary Pharmacy Act (TÄHAV), which has been in force since 1st March 2018, has raised some questions regarding the treatment of keratoconjunctivitis. Since the TÄHAV came into effect, the main problem for practitioners lies in the fact that, so far, keratoconjunctivitis in dogs and cats has frequently been treated with antibiotic agents that have no approval for use in these animal species in Germany or even contain reserve antibiotics belonging to the gyrase inhibitor group. We have therefore compared the infectious causes of these diseases with current diagnostic data and show the level of resistance of the pathogens involved to selected agents.
(Kerato)conjunctivitis in cats
Primary infections in cats are mainly caused by feline herpesvirus-1 (FHV-1), closely followed by chlamydia. FHV-1 multiplies in the epithelial cells – including those of the cornea – which is why damage to the cornea (keratoconjunctivitis) with possible subsequent ulceration can occur. These ulcers are almost always secondarily infected with bacteria. Ulcers are a complication that is less frequently seen in chlamydia infections. Chlamydia felis is highly contagious and is shed in eye and nose secretions. Mycoplasma felis is another primary but much rarer cause of conjunctivitis in cats. PCR is the usual detection method for these three primary pathogens. We determine all three pathogens in one eye profile. The material to be submitted is an eye swab rich in cells obtained with a dry swab and sent without transport medium!
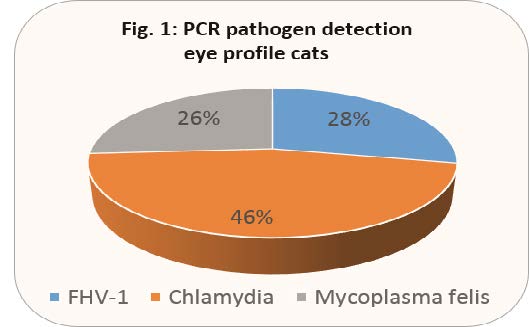
Fig. 1 shows the detection frequency of the individual pathogens from the second half of 2017 (total number of samples: 481). PCR detection was positive in 46% of cases, with 39% of all samples tested showing a mono-infection with one of the pathogens and 7% of the samples showing a multiple infection.
Multiple infections most often occurred in combination with mycoplasma, a result consistent with studies in other countries. According to one study, co-infections are associated with more severe clinical signs than single infections. Although FHV-1 is considered to be the most common primary pathogen of the clinical picture mentioned, it cannot be detected in a prolonged infection because the virus is only shed for a maximum of 10 days after infection. Thus, chlamydia have the highest detection frequency in PCR detection, as PCR is often only performed in case of a chronic illness or after the first treatment failed. However, like all herpes viruses, FHV-1 cannot be eliminated, but retreats to different body parts and remains latent there. Triggered by other diseases, stress or corticosteroid treatment, virus shedding can occur again at any time and also lead to recurring clinical signs.
Mild forms of FHV-1-related keratoconjunctivitis are often self-limiting. In severe clinical cases or in case of persistent relapse, however, oral or topical antiviral medication should be used. For chlamydia and mycoplasma, there are no methods established for routine resistance testing. In therapy, antibiotics that have a known effect against these pathogens are used. This also conforms to the TÄHAV. Tetracyclines, chloramphenicol or gyrase inhibitors – used three to four times a day as eye ointment or drops – have a good effect against these pathogens. In case of chlamydia, topical therapy is sometimes not sufficient to eliminate the pathogens in cats, especially if the excretion also occurs via other mucous membranes, e.g. of the genital tract. In these cases, additional oral treatment with doxycycline for 30 days is indicated, especially in multi-cat households. Here, it is recommended to treat all cats of the household with doxycycline. Because of the strong side effects of doxycycline alone, such an approach should be backed up by PCR in any case.
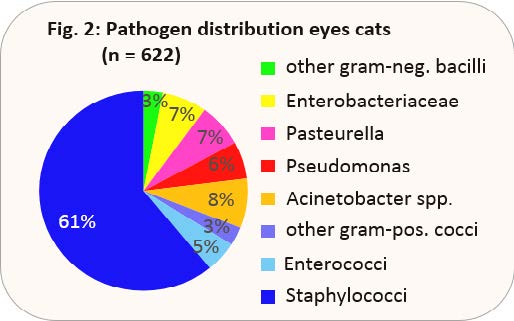
Frequently, the original primary infection is secondarily colonised by facultative pathogenic, aerobic bacteria. Particularly FHV-1-related ulcers are often secondarily infected with bacteria. 866 swab samples of eye swabs from 2017 were bacteriologically evaluated. Fig. 2 shows the pathogen distribution of these samples. Staphylococci take up by far the largest part of detected pathogens, but only 4% of these ocular staphylococci show multiresistance.
In mild clinical cases, empirical topical treatment is often sufficient, since local application achieves a high concentration of active ingredients directly at the site of infection. Cytology can provide information on whether there are indications of inflammation with bacterial involvement. Sampling should be done before the application of cleaning substances or fluorescein. Swabs for bacteriological examination with resistance test must be sent with transport medium! According to a new study conducted by the University of Berlin together with Laboklin, moistening the swab with sterile physiological saline solution improves the detection rate of gram-positive pathogens. The swab should not be placed directly in the pus, as the pathogens contained therein are in a phagocytised form and may therefore not be cultivable.
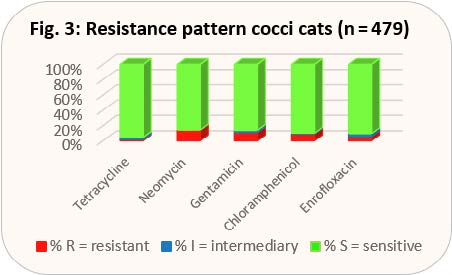
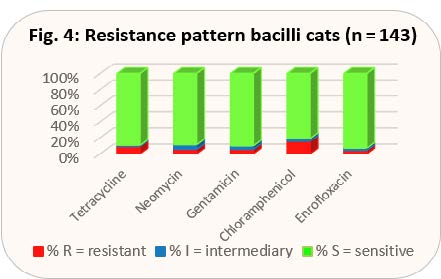
Although Diff-Quick staining prepared in the practice from a second swab for cytology does not give any indication of the presence of gram-positive or gram-negative pathogens, it does, however, indicate whether bacilli or cocci are present in the preparation. When taking a closer look at the distribution of pathogens, it becomes clear that bacilli always need to be classified as gram-negative and cocci, with the exception of Acinetobacter spp., always as gram-positive. However, Acinetobacter spp. do not play a pathogenic role in conjunctivitis. That is why we have each combined bacilli and cocci in the presentation of the current resistance behaviour, since the cytological results can thus be transferred directly to the empirical selection of an antibiotic (see Figs. 3 and 4).
For cats, there are topical preparations approved in Germany and available on the market for the active substances tetracycline, neomycin and gentamicin (source: Vetidata, December 2018).
- Fig. 1: PCR pathogen detection eye profile cats
- Fig. 2: Pathogen distribution eyes cats (n = 622)
- Fig. 3: Resistance pattern cocci cats (n = 479)
- Fig. 4: Resistance pattern bacilli cats (n = 143)
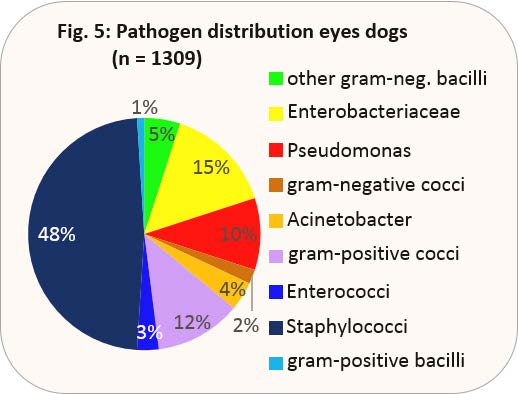
- Fig. 5: Pathogen distribution eyes dogs (n = 1309)
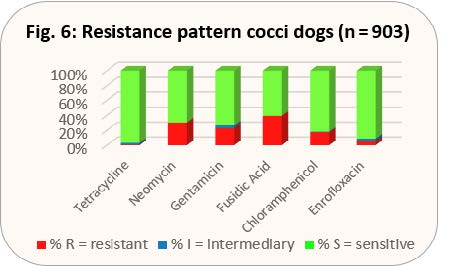
- Fig. 6: Resistance pattern cocci dogs (n = 903)
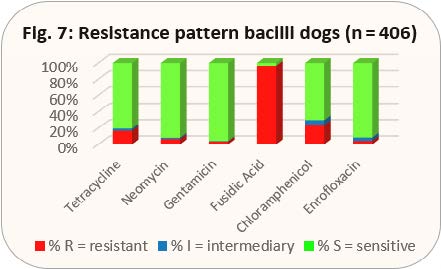
- Fig. 7: Resistance pattern bacilli dogs (n = 406)
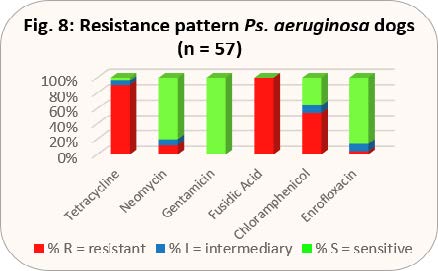
- Fig. 8: Resistance pattern Ps. aeruginosa dogs (n = 57)
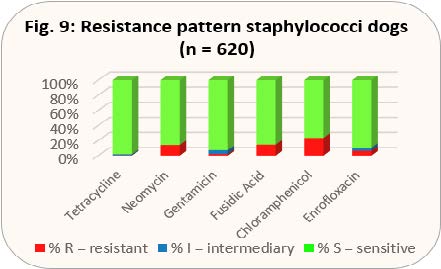
- Fig. 9: Resistance pattern staphylococci dogs (n = 620)
According to the new TÄHAV, there is no obligation to perform an antibiogram for these antibiotics. Chloramphenicol is available in a formulation approved for use on animals in other EU countries. Floxal® eye ointment with the active ingredient ofloxacin is a human medicine preparation that, so far, has been very popular in the small animal practice. As ofloxacin is not tested in our antibiogram, the results for enrofloxacin have been included in the evaluation instead. Looking at the results, it can be seen that in cocci tetracycline is even better than gyrase inhibitors, while the level of resistance in bacilli is similar. Both antibiotics also reach chlamydia and mycoplasma well. Thus, an approved preparation is available, since for Floxal® ointment, the new TÄHAV prohibits any extra-label use. If an exception to this prohibition can be justified, it is obligatory to perform an antibiogram in any case (use of a gyrase inhibitor and extra-label use according to level 3 §56a AMG (German Drug Law)). Another advantage of tetracycline is that it has an epithelising effect on corneal ulcers by inhibiting the matrix metalloproteinases produced by the corneal epithelium and promoting collagen lysis. Chloramphenicol ointment would also have a good effect, at least against cocci, and is also effective against chlamydia and mycoplasma, but according to the new TÄHAV it is subject to the obligation to perform an antibiogram, as it is veterinary medicine imported from abroad (level 3 of the Cascade Rule §56a AMG). The approved eye ointment containing neomycin basically has the disadvantage that it also contains a corticosteroid. As (kerato)conjunctivitis in cats is usually infectious, the immunosuppression triggered by the steroid may have a massive negative effect on the infection. Recently, eye drops containing gentamicin have been approved for cats in Germany. They do not contain steroids. In bacilli, the level of resistance is better than that of tetracycline.
(Kerato)conjunctivitis in dogs
In contrast to cats, viruses, chlamydia or mycoplasma play only a minor role as primary pathogens of keratoconjunctivitis in dogs. A look at the results of the eye profile from the second half of 2017 (PCR detection for canine herpesvirus, chlamydia and mycoplasma) shows: only 6.4% of 108 samples examined were positive. Multiple infections did not occur and mycoplasma was most frequently detected with 4.6%.
In dogs, conjunctivitis is often associated with atopy. There are neither clinical nor cytological signs of a real infection (no purulent discharge, no phagocytised bacteria). In addition to the increased presence of keratinised epithelial cells, lymphocytes and eosinophils, there are extracellular bacteria and malassezia that are also found in cytology. These pathogens occur even more often than in healthy dogs. However, antibiotic treatment is not indicated here. For some time now, there have been eye drops containing a corticosteroid that are approved for this field of application in dogs.
In dogs, infections of the conjunctiva are mostly secondary, e.g. in connection with keratoconjunctivitis sicca. The pathogen distribution is shown in Fig. 5.
For this purpose, 1396 swab samples of eye swabs from 2017 were evaluated. Staphylococci take up by far the largest part of detected pathogens; only 1% of these ocular staphylococci show multiresistance.
The resistance pattern in dogs is shown in the same way as it was done for cats (see explanation there, Figs. 6 and 7). In addition to the approved preparations for cats, there is an approved eye ointment with fusidic acid for dogs.
Infected corneal defects play a special role in dogs. They can quickly become deep ulcers and it is generally necessary to act quickly to protect the eye from massive damage. The pathogens involved are usually staphylococci or Pseudomonas aeruginosa. Treatment should be based on a bacteriological examination with resistance test, but as a start, gentamicin or ofloxacin can be used topically until the result is received. According to the new TÄHAV, however, a good justification is needed for any extra-label use of ofloxacin, and there is an obligation to perform an antibiogram (see the chapter on cats).
With the results shown in Figs. 8 and 9, gentamicin would be preferable to gyrase inhibitors.
Additionally, according to a recent study, prolonged administration of ofloxacin leads to an altered conjunctival flora with an increase in resistance to this antibiotic.
Another study showed that ofloxacin was not superior to aminoglycoside in the long-term healing of the disease. Thus, the gyrase inhibitor could also be avoided in this disease, as it should only be used selectively and can promote the rapid spread of resistances. Topically applied corticosteroids should be avoided when treating corneal ulcers.
Conclusion
In cats, PCR should be used regularly and at an early stage to clarify primary infectious agents, as FHV-1 infections and chlamydia may require measures beyond topical antibiotic therapy. Primary pathogens often cause a secondary infection with facultative pathogenic agents. Multi-resistant ocular pathogens are rare. There are preparations approved for dogs and cats for which there is no obligation to perform an antibiogram according to the new TÄHAV and which show excellent efficacy in various clinical pictures. According to antibiotic guidelines, a bacteriological examination with resistance test should be carried out in case of relapses and when changing antibiotics because of treatment failure. This is understood as a responsible approach to antibiotics. There is almost no need to use gyrase inhibitors; here, an antibiogram according to TÄHAV would be required in any case.