The work-up of equine respiratory diseases is based on anamnesis, clinical examination, endoscopic findings and pulmonary function tests; additionally, laboratory examinations, such as cytology of sample material from the respiratory tract (TBS – tracheobronchial secretion and BAL – bronchoalveolar lavage), are also important elements in the diagnostic process. As ever, the value of laboratory tests stands and falls with selecting the suitable sample material and its optimal preparation.
TBS and/or BAL examination is indicated for classic signs of respiratory tract diseases such as cough, pathological breath sounds, nasal discharge and dyspnoea. However, exercise intolerance is also one of the indications since the clinical signs can be very subtle, especially in mild to moderate equine asthma (formerly: Inflammatory Airway Disease (IAD)).
The collection of TBS and BAL was recently described in great detail by Schwarz and Kühn with helpful tips for practical application. But when is it preferable to examine TBS, and when is it better to obtain BAL? What is the significance of the findings from the different sample materials?
Ideally, both sample materials should be obtained. For bacteriological examinations, material from the trachea is preferred, so if bacterial diseases are suspected (e.g. fever), TBS should always be taken. BAL is even contraindicated in suspected bacterial pneumonia. However, BAL is much more suitable for cytological examination because its findings correlate better with the pathophysiology than those of TBS. Furthermore, since the cytological picture of TBS is not representative of the deeper airways, BAL is the method of choice for the cytological study of diffuse lung diseases.
Correct sample preparation is as crucial for successful diagnosis as sample collection. Unfortunately, cells degenerate very quickly in washing fluids. Therefore, even if samples are sent to a laboratory for evaluation, cytological preparation should be performed immediately from the freshly obtained sample material. If the smears are only made in the laboratory, the cells can often no longer be assessed after only a few hours of transport/ storage, and the significance of the cytological findings decreases rapidly.
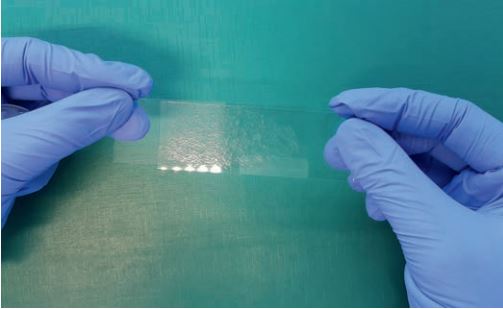
If the TBS can be obtained without washing, smears are prepared directly from the mucus. For this purpose, a drop of sample is placed on a microscope slide, a second slide is placed on top of it without pressure and quickly pulled over the first one, or both slides are spread apart in opposite directions so that the sample material is distributed on the slide “like butter on a loaf of bread“ (Fig. 1). If the TBS was obtained by washing, the material is first centrifuged at low rotation speed (200 – 300 g), the liquid is decanted, and finally, a drop of the sedimented mucus is deposited as described above.
The BAL fluid is always cell-poor, so enrichment preparations must be made for cytological evaluation. The simplest method is to perform sediment smears: a part of the sample is centrifuged at low speed, analogous to tracheal lavage samples and the supernatant is largely decanted. The cell pellet is resuspended with the rest of the liquid and spread using the blood smear technique. A slight suspension drop is placed on one end of a microscope slide. A second slide is positioned in front of the drop at an angle of about 45° and move towards the drop until the liquid spreads over the entire edge. Finally, spread the sample on the slide by pushing the slide forward vigorously and evenly. The highest quality preparations for assessing cell-poor fluids are produced by cytocentrifugation. Special inserts are available for some centrifuges for this purpose, but they are only worth buying if used frequently. However, it is also possible to construct a homemade “sedimentation chamber“ (Fig. 2): a 2 ml syringe is pressed firmly onto a microscope slide with strong clamps over a filter paper with a hole of the same size precisely at the opening of the syringe. The sample material is poured in through the cone of the syringe. The filter paper slowly absorbs the liquid, enriching the sample with cells on the hole area of the slide. To avoid in vitro multiplication of bacteria, the chamber should be kept in the refrigerator during sedimentation.
It is essential to dry the preparations as quickly as possible to preserve the cells. Especially with viscous material, the drying process can be accelerated by placing the smears in an incubator or on a warm surface. Careful blow-drying with lukewarm air is also possible. For shipment to a laboratory, the dry, unstained slides are packed in shipping boxes, representative aliquots of the collected specimens are enclosed in tightly sealed, uncoated test tubes. The liquid sample material should reach the laboratory as quickly as possible and cooled (possibly add a cold pack).
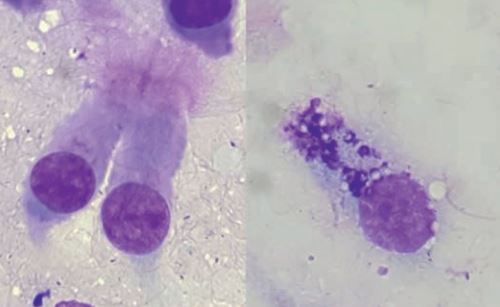
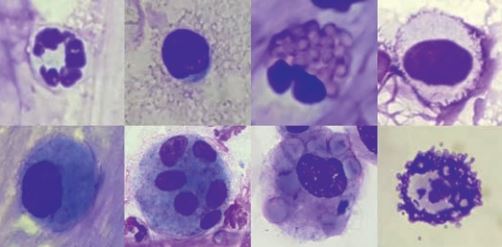
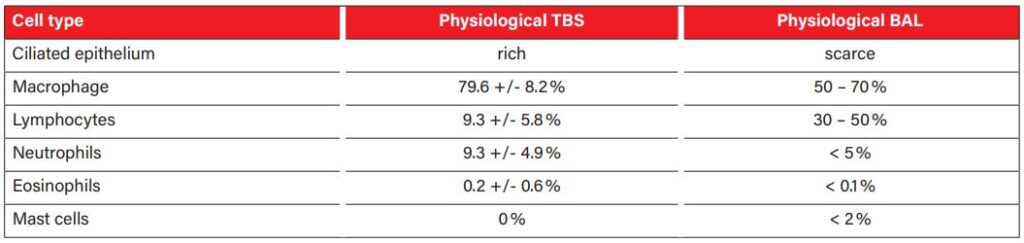
Preparations can be stained with commercial Romanowsky-type rapid stains, but at least one smear should be stained with a specific stain for mast cell granules, such as toluidine blue. These granules are easy to miss in respiratory tract samples, although they are diagnostically very significant (Fig. 4). The physiological cell pattern of TBS and BAL reflects the anatomical structure of the respective sections of the respiratory tract. In healthy animals, the cytological smears are cell poor, with few tissue cells (ciliated epithelium and goblet cells, fig. 3) and a small number of inflammatory cells (mainly macrophages and lymphocytes, fewer neutrophils, eosinophils and mast cells, fig. 4).An overview of the cellular composition of TBS and BAL in asymptomatic horses is shown in table 1.
There are hardly any reference values for the cellular composition of TBS in the literature. The often-quoted statement that a percentage of more than 20% neutrophils is indicative of an inflammatory process should be considered with caution. Several studies have shown that even clinically healthy horses can have a significantly increased proportion of neutrophils in the TBS under certain conditions. Causes discussed include hay feeding in the form of round bales, being outdoors in cold weather and intensive training. The cell composition of BAL is much less dependent on environmental factors is, therefore, better suited for the diagnosis of disseminated diseases, especially of the deeper respiratory tract. Of course, the most significant results are obtained by examining TBS and BAL simultaneously.
-
Group of young horses on the pasture
Picture Credits: Dr. Maria Christian
-
Fig. 1: Squeeze technique
Picture Credits: Laboklin
-
Fig. 2: A sedimentation chamber built from a slide, a perforated filter paper, a syringe and 2 strong clamps.
Picture Credits: Laboklin
-
Fig. 3: Romanowsky-type rapid stain. On the left: ciliated epithelium. On the right: a goblet cell.
Picture Credits: Laboklin
-
Fig. 4: Inflammatory cells; above left to right: neutrophil, lymphocyte, eosinophil, mast cell (Romanowsky rapid staining); below left to right: inactivated macrophage, multinucleated macrophage, erythrophagocytosis, mast cell (toluidine blue).
Picture Credits: Laboklin
-
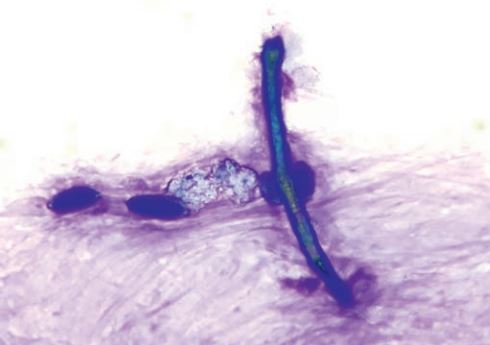
Fig. 5: Elongated, double-walled fungal hypha and 4 pollen grains. Rapid romanowsky-type staining
Picture Credits: Laboklin
-
Tab. 1: TBS and BAL physiological cell composition
Picture Credits: Cian et al. 2015
-
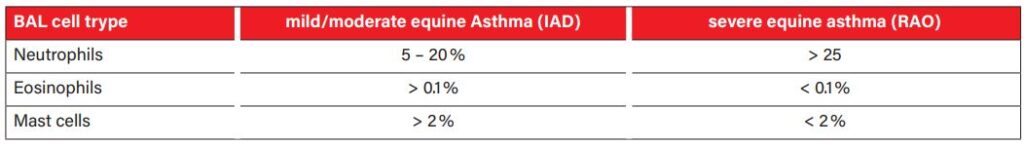
Tab. 2: Cellular composition of BAL in equine Asthma
Picture Credits: Cian et al. 2015
-
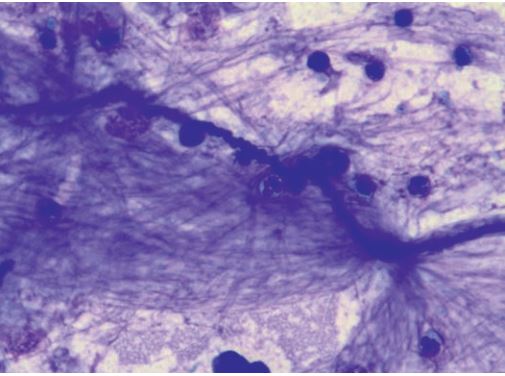
Fig. 6: Curschmann spiral, Rapid romanowsky-type stain.
Picture Credits: Laboklin
In respiratory tract diseases, changes occur both in cell content and the proportion of different cell types. In addition, cytological examination looks for structures such as inhaled fungal elements (fig. 5), plant particles and pollen (fig. 5) or infectious agents such as bacteria. Curschmann‘s spirals (fig. 6) are thick mucus secretions from the small airways and indicate poor mucociliary clearance.
In the differentiation of sterile respiratory inflammations, such as mild to moderate equine asthma (formerly IAD) and high-grade equine asthma (formerly: recurrent airway obstruction (RAO)), the determination of the cellular pattern of the LBA is helpful (Tab. 2). Both forms of asthma result in an increased percentage of neutrophils in the sample. In mild to moderate equine asthma, the increase is mild (5 – 20% neutrophils), and an increase in mast cells (> 2%) and eosinophils (> 0.1%) may also be found. High-grade equine asthma is associated with a marked increase in neutrophils (> 25%); almost exclusively, neutrophils are found in many cases. Septic inflammation usually causes very high cell counts with a very high proportion of neutrophil granulocytes. Without detecting phagocytosed bacteria, the cellular picture may look precisely like a high-grade sterile inflammation. Therefore, it is essential to always interpret cytological findings in combination with the clinical history and presentation. If a bacterial disease is clinically suspected, a bacteriological examination from the TBS should always be performed. Macrophages that have phagocytosed erythrocytes (Fig. 4) or contain their degradation products (haemosiderin or haematoidin) indicate the presence of bleeding in the lungs. Mild haemorrhages may occur in various diseases and may be caused, for example, by severe coughing attacks. However, prominent blood inclusions may indicate the presence of exercise-induced pulmonary haemorrhage (EIPH). Of course, a haemorrhage has to be distinguished from a blood clot-related to sampling. This is ensured by evaluating the preparations made from the freshly obtained sample. If there is also evidence of erythrocyte degradation here, there is a haemorrhage.
In some rare lung diseases, such as equine multinodular pulmonary fibrosis (EMPF), a pathohistological examination of the lung tissue is necessary for the diagnosis, as the cytological findings of the BAL are not specific. However, PCR can analyse the BAL for EHV-5, which is associated with EMPF.
Cytology of TBS and BAL makes a valuable contribution to the diagnosis of respiratory disease and provides information on the severity of the disease and therapeutic approaches. The selection of appropriate specimen material and the provision of well-assessable cytological preparations are crucial for significant findings.
Dr Maria Christian