Hepatic diseases are quite common in equids. In addition to poisonous plants, toxins, parasitoses and neoplasms, they can also be caused by bacterial or viral infectious agents. Many aetiologies have already been studied sufficiently, but the cause of the disease known as Theiler’s disease or serum hepatitis has remained largely unexplained for over a century. Since 2012, research has focused on the following four viruses as possible causes of hepatopathies: equine hepacivirus (EqHV), equine pegivirus 1 (EPgV), Theiler’s disease-associated virus (TDAV) and equine parvovirus-hepatitis virus (EqPV-H). EqHV, a flavivirus that was first detected in equine serum in 2012, can cause both an acute and a chronic persistent course of infection. It is not yet known whether infection with EqHV, which is thought to show liver tropism, always leads to clinical signs.
So far, no affinity to hepatic tissue has been confirmed for EPgV, but performance losses have been described. TDAV was given its name by mistake after an outbreak in 2013 with signs of acute hepatitis. Once it was detected that TDAV did not cause the outbreak but only occurred as a co-infection together with equine parvovirus, it was renamed as equine pegivirus 2.
The equine parvovirus hepatitis virus (EqPV-H), which, thanks to Thomas Divers’ team, has now been identified as the cause of Theiler’s disease, will be discussed in more detail below.
EqPV-H is a single-stranded, non-enveloped DNA virus that belongs to the genus Copiparvovirus. The highest viral load of EqPV-H is found in the liver, which indicates hepatotropism.
So far, two different modes of transmission are assumed:
One is the administration of products made from horse serum containing equine parvovirus. These include tetanus antitoxin, botulinum antitoxin, stem cell preparations and equine plasma products in general.
On the other hand, EqPV-H outbreaks also occur in horses that have not received a biological preparation in the past. In this case, transmission between horses or spread by insects is assumed, but this is currently still being researched.
Clinical signs of EqPV-H infection occur about 4 – 10 weeks after the administration of a biological product infected with the virus. The course of the disease ranges from asymptomatic to fulminant liver failure. Acute hepatitis is characterised by lethargic behaviour with accompanying anorexia and icteric mucous membranes (Figure 1). Some of the infected horses show neurological signs, such as manic behaviour, head pressing and also ataxic movement, as a result of hepatic encephalopathy. Moreover, colic, recumbency (Figure 2) and death within 72 hours have been described.
In addition to a detailed clinical history and a thorough general examination, the diagnostic work-up of patients with liver disease also includes a blood test.
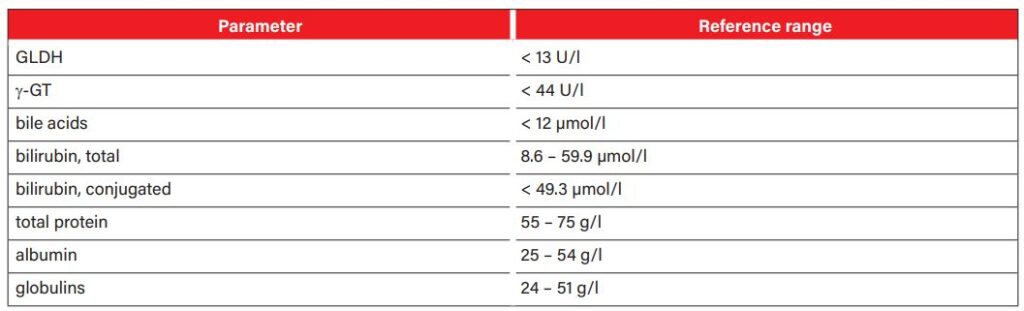
The following laboratory parameters may indicate acute liver dysfunction in infected horses:
The activity of the liver-specific enzymes gamma-glutamyl transferase (γ-GT) and glutamate dehydrogenase (GLDH) is usually increased in serum. However, the extent of the increase does not correlate with the functional abnormalities. While γ-GT is mainly located in the membrane structures of the bile duct system, GLDH is bound to the mitochondria of the hepatocytes. Both enzymes have a half-life of about 3 days. Alkaline phosphatase (AP) is found in the mitochondria of many organs, so it should not be interpreted as a liver-specific parameter. Aspartate aminotransferase is also not liver-specific as it can also be found in muscle cells and its activity increases with muscle damage. In addition, horses may also have increased levels of bile acid. Hepatocytes synthesise bile acids from cholesterol. They are continuously secreted into the duodenum where they help to digest fats. In hepatopathy, bile acids accumulate and their increase can be measured in the blood. Bile acid values above 12 µmol/l are considered an early diagnostic marker for functional liver failure. Neurotoxic ammonia, which is produced during protein digestion, also increases in the blood in case of liver dysfunction (resulting in hepatoencephalic syndrome). However, a laboratory-diagnostic determination of the ammonia level is only possible if the sample material reaches the laboratory quickly (within 30 minutes), which makes it difficult to use in practice. If hyperbilirubinaemia is present, it is advisable to classify it more precisely as conjugated and unconjugated bilirubin in order to narrow down the cause of jaundice (pre-hepatic, hepatic, post-hepatic). Albumin is low in about 18% of horses with liver failure, whereas globulins are elevated in about 64% of horses. Table 1 summarises the relevant laboratory parameters for the work-up of patients with liver disease.
An ultrasound scan of the liver from both sides of the body and a liver biopsy complete the diagnosis of hepatic disease.
-
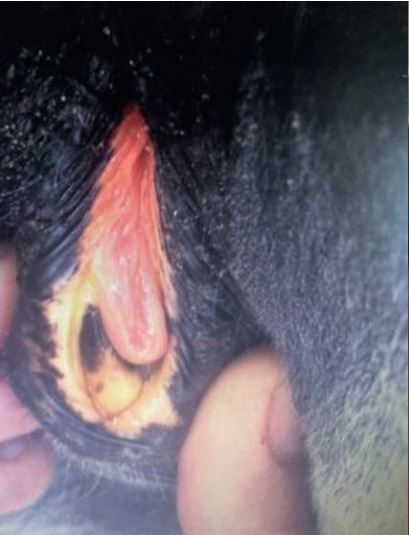
Fig. 1a: Icteric mucosa of a horse with hepatic disease
Picture Credits: Adobe Stock
-
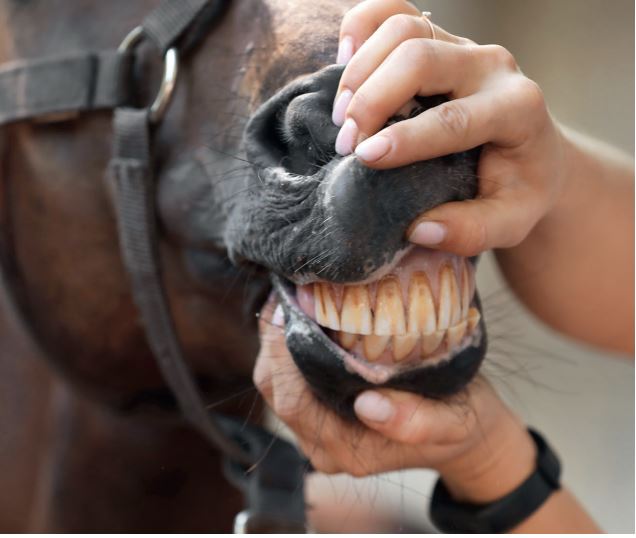
Fig. 1b: Icteric mucosa of a horse with hepatic disease
Picture Credits: Adobe Stock
-
Fig. 2: Possible sign of acute serum hepatitis: recumbency in a horse
Picture Credits: Adobe Stock
-
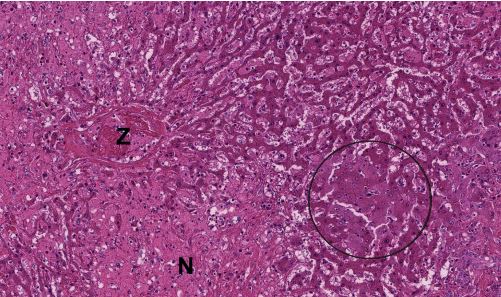
Fig. 3: Histopathological preparation of a liver biopsy: hepatic necrosis in a horse Z = central vein, N = necrosis, circle = markedly swollen hepatocytes
Picture Credits: Laboklin
-
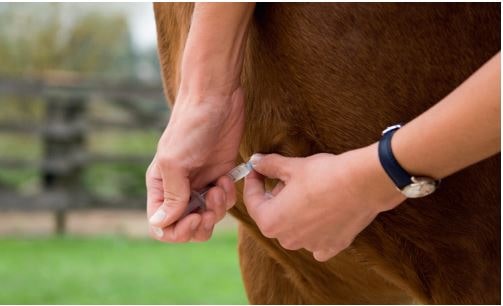
Fig. 4: Prevention: administration of a biological preparation which has been tested negative for EqPV-H
Picture Credits: Adobe Stock
- Tab. 1: Laboratory parameters relevant for the work-up of patients with liver disease Laboratory parameters must be interpreted in the context of medical history, clinical examination and further tests.
The clinical history is essential for making a diagnosis. If an adult horse shows signs of acute liver failure and if, according to the anamnesis, it was administered an equine biological product about 4 – 10 weeks before, equine parvovirus should be considered as a possible differential diagnosis.
The virus is detected by PCR from blood or liver tissue. A liver biopsy with subsequent histopathological examination may be useful to identify or exclude additional causes of hepatitis. Pathology shows a shrunken liver with a flattened edge. The other internal organs do not show any striking abnormalities. Histopathologists describe varying degrees of centrolobular hepatitis and hepatocellular necrosis (Figure 3). Partial vacuolisation of the remaining hepatocytes can be observed.
There are many possible differential diagnoses for equine liver failure. In addition to viral infectious agents, toxic, parasitological or bacterial aetiologies should be considered, too. Cholestasis or lipidosis may also be possible causes of liver disease.
There is no specific treatment for horses suffering from serum hepatitis. In asymptomatic horses, monitoring of liver enzymes is recommended. Horses with acute hepatitis usually require intensive monitoring. Depending on the clinical signs, infusions, feeding by nasogastric tube, the administration of NSAIDs, antibiotic treatment and other measures are necessary.
Dietary measures in liver diseases mainly focus on reducing the burden on the liver caused by protein breakdown in the intestine. The amount of protein should be limited to what is strictly necessary and divided into several small meals. To ensure an adequate energy supply in patients with liver disease even with reduced protein intake, the food ration must contain sufficient carbohydrates. Corn flakes or extruded cereals (0.3 kg/100 kg bdw per meal) are suitable for this. Supplementation with linseed extraction meal or brewer’s yeast provides additional essential amino acids. In addition, feeding lactose (0.2 g/kg bdw) or lactulose (0.5 – 1g/kg bdw) 3 times a day also relieves the liver.
Once the infection is overcome, the prognosis is favourable. No late effects have been described.
Prevalence is estimated to be between 3% and 17%. It is currently assumed that only about 2% of infected horses develop clinical signs. However, research on the epidemiology of equine parvovirus is still in its early stages. As there is no vaccine for equine parvovirus at present, the only way to prevent it is to only administer preparations that have been tested negative for EqPV-H.
Products which have been reviewed and approved by the USDA APHIS (U.S. Department of Agriculture, Animal and Plant Health Inspection Service) bear a VLN/VPN and the product code number.
There is no known zoonotic potential of equine parvovirus.
Conclusion
If we, as veterinarians, are presented with an adult horse showing signs of acute liver failure that was administered an equine biological product about 4 – 10 weeks ago, we should consider equine parvovirus as an important differential diagnosis. The exact modes of transmission will continue to be a fundamental part of future research in order to determine possible prevention and control measures and to be able to draw conclusions about how relevant it is to isolate affected horses.
Dr. Carla Gerhard