The most important lungworm species belong to the helminth superfamily Metastrongyloidea, the adults of which live in the lungs of their vertebrate hosts. Aside from these parasites, there are also members of the family Trichuridae, which can also be found in the respiratory tract. This factsheet will cover the most common European species as well as the different diagnostic methods.
Angiostrongylus (A.) vasorum, Crenosoma (Cr.) vulpis, Oslerus (O.) osleri and Filaroides (F.) hirthi mainly infect wild canids and domestic dogs, Aelurostrongylus (Ael.) abstrusus and Troglostrongylus (T.) brevior use wild felids as well as domestic cats as definite hosts.
Capillaria (C.) aerophila (syn. Eucoleus aerophilus) exhibits a low host specifity. This species can be found mainly in foxes and hedgehogs, but dogs, cats and some other species, including humans (zoonosis!), also can be affected.
Life cycle
The following lungworm species present with an indirect life cycle using intermediate hosts:
- Cr. vulpis
- Ael. abstrusus
- T. brevior
- A. vasorum
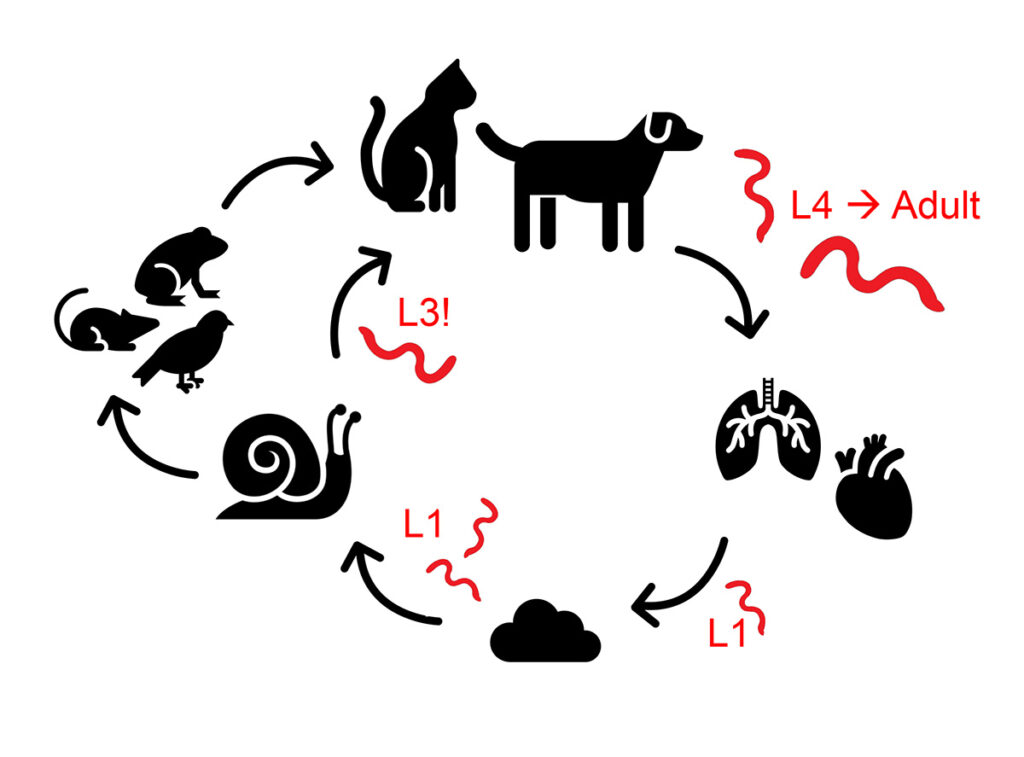
In Cr. vulpis, Ael. abstrusus and T. brevior, adult worms can be found in trachea, bronchi, bronchioli and alveoli, depending on the species. Adult females release their eggs into the respiratory tract, where the first stage (L1) larvae hatch. The L1 larvae will be coughed up, swallowed and then shed via the faeces. Once in the environment, a variety of slug and snail species become infected (obligatory intermediate hosts), and the larvae develop into L3 larvae, which are infectious for the vertebrate hosts. The definite vertebrate hosts will ingest these parasite larvae by eating either the intermediate hosts directly or the transport hosts (e.g. birds, rodents, amphibians or reptiles), which have ingested the slugs and snails themselves (Figure 1).
In contrast, the adults of A. vasorum (also known as French heartworm) live in the pulmonary arteries as well as the right side of the heart. The eggs produced by the females are transported to the lung via the blood. Once in the lung, the larvae (L1) hatch and penetrate the walls of the alveoli. From there, the further development proceeds as described above.
The following parasites have a direct life cycle (without an intermediate host):
- O. osleri
- F. hirthi
- Possibly also C. aerophila
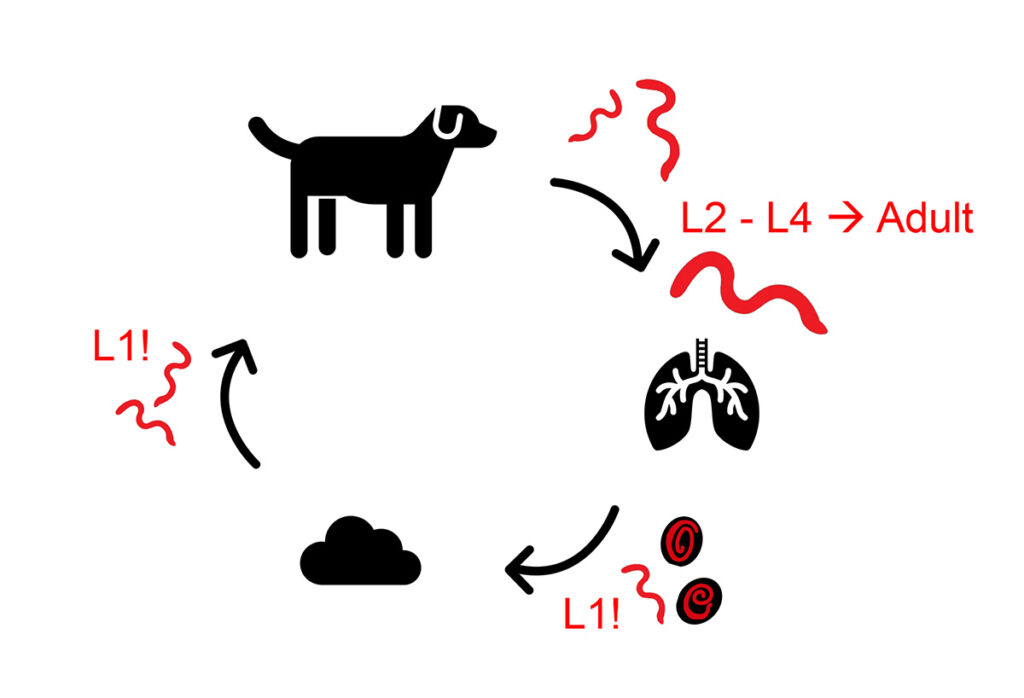
The adult worms of O. osleri cause visible nodules in the mucosal epithelium of trachea and bronchi. From there, females release eggs, which already contain an infectious L1 larva, into the tracheal lumen. The adults of F. hirthi live inside the lung parenchyma and infectious L1 larvae are coughed up, swallowed and then shed with the faeces. These larvae are transmitted via the faecal-oral route, often horizontally at a young age. Autoinfections are common. Dogs positive for O. osleri- and F. hirthi should be isolated and all dogs who were in contact with them should also be treated, because of the direct infectivity of the shed L1 larvae (Figure 2).
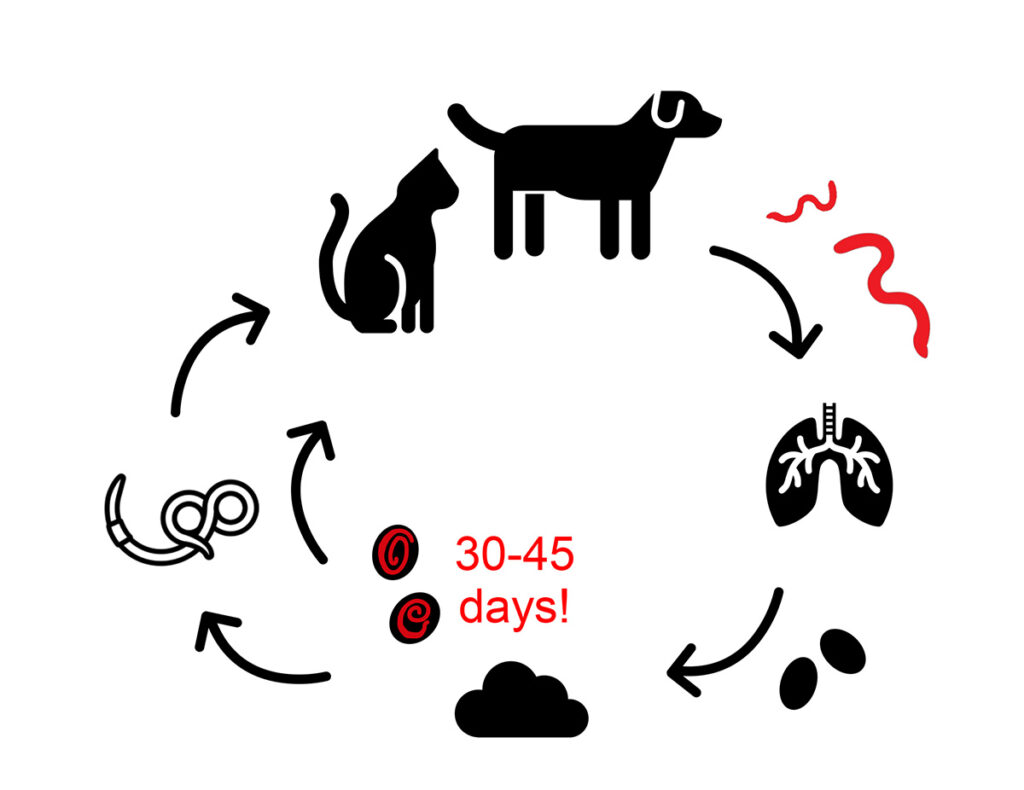
The adult stages of C. aerophila are embedded in the submucosa of trachea, bronchi and bronchioli. There, they release eggs, which are coughed up, swallowed and shed in the faeces. Depending on the environmental conditions, the development into infectious larvae (in embryonated eggs) takes 30-45 days. Earthworms play an important role in the transmission of C. aerophila, however, it is not yet known if earthworms are transport- or intermediate hosts (Figure 3).
Important for all lungworm species: definite hosts (vertebrates like dogs and cats) become infected after the ingestion of the infectious stage. This depends on the parasite species, either the intermediate or transport host is eaten, or the transmission happens via the oro-faecal route. After ingestion, the worm larvae penetrate the intestinal wall and migrate either via the blood- and lymph vessels, or through the coelomic cavities towards the lung. There, they mature into adults and become sexually active.
Clinical Symptoms
Clinical symptoms can vary and are a consequence of the tissue damage caused by the adults and migrating larvae. The severity of the disease is, among other things, dependent on the worm species and -count. Younger animals are affected more frequently and they also present with more severe symptoms, owing to a smaller lung volume and still developing immunity.
Clinical inapparent infections without any symptoms are possible, these animals can shed lungworm larvae or Capillaria eggs and these can be detected accidentally during routine faecal examinations.
On the other hand, mild to severe respiratory symptoms are likely, presenting as:
- Coughing
- Nasal discharge
- Tachypnoe
- Dyspnoe
In addition, infections with A. vasorum can cause coagulation disorders, pleura effusion as well as cardiovascular and neurological symptoms.
Fatalities are possible.
- Source: Dr. Michaela Gentil
-
Figure 1: Life cycle of lungworm species with indirect development, infectious stage is marked with „!”
Source: Laboklin
-
Figure 2: Life cycle of lungworm species with a direct
development, infectious stage is marked with „!”
Source: Laboklin
-
Figure 3: Life cycle of Capillaria aerophila, infectious stage is marked with „!”
Source: Laboklin
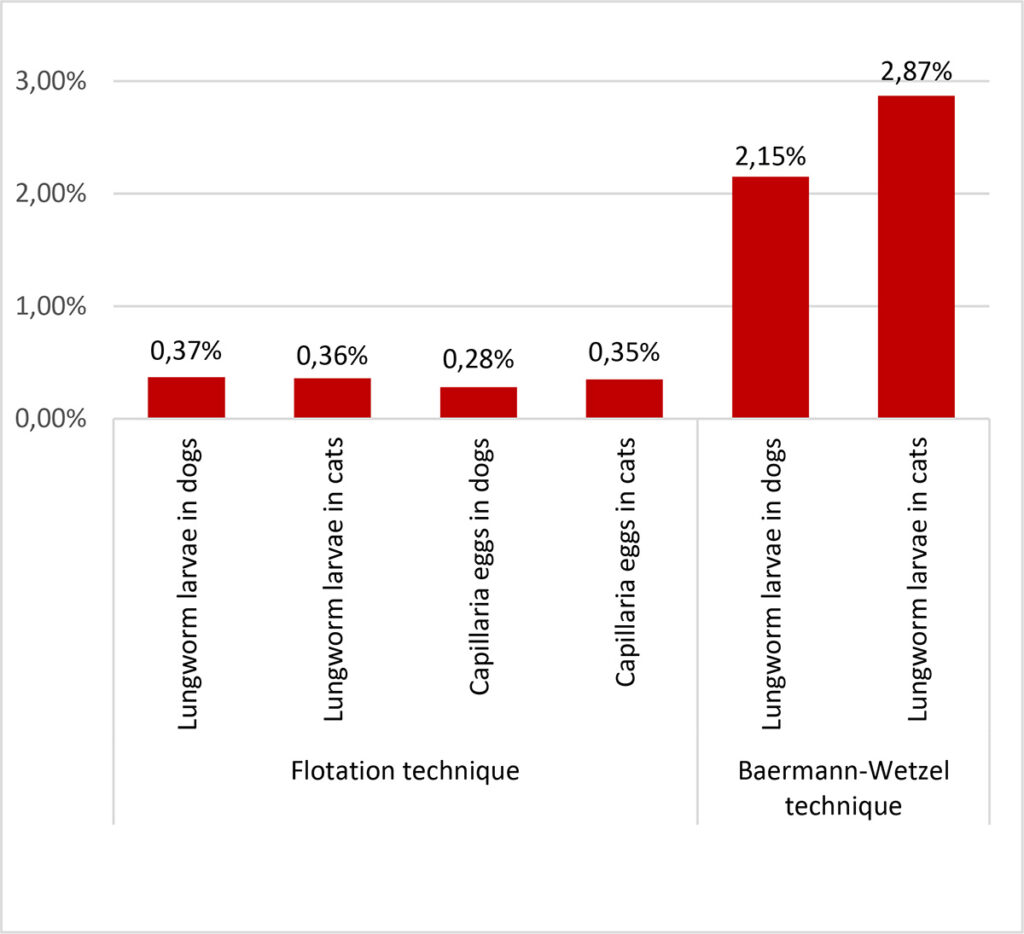
- Figure 4: Frequency of occurrence of lungworm larvae and Capillaria eggs using flotation (NaCl-glucose-solution [ρ = 1,3]) and Baermann-Wetzel technique for larval migration in dogs (n = 97882 respectively 5496) and cats (n = 23869 respectively 2160) in Germany during 2021 – 2022.
-
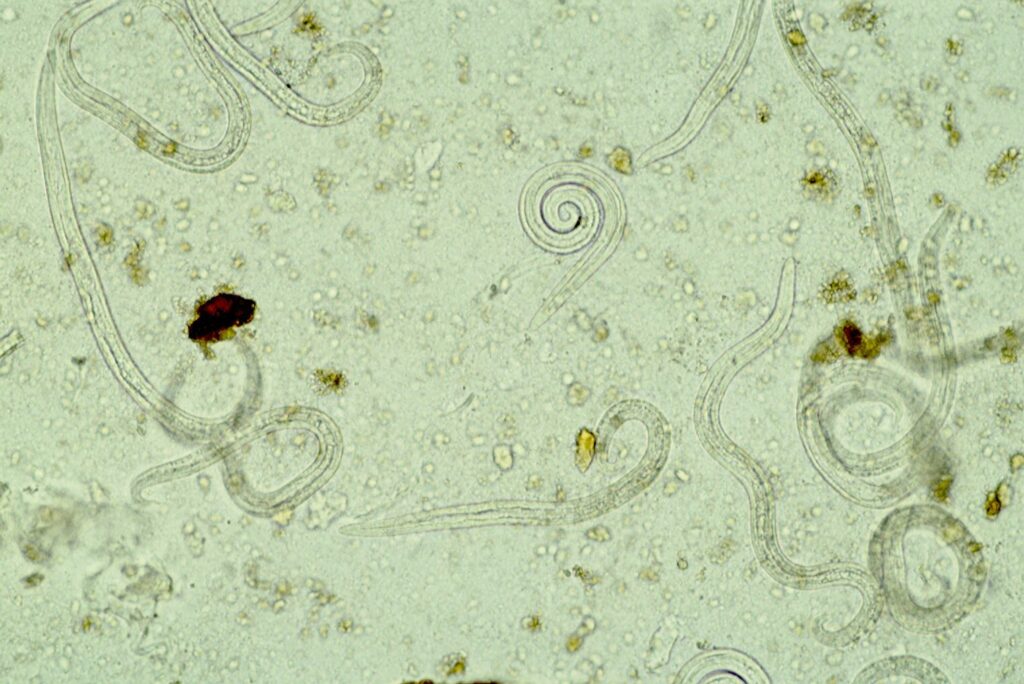
Figure 5: Lungworm larvae seen with the Baermann-Wetzel technique for larval migration, 20x magnification
Source: Laboklin
-
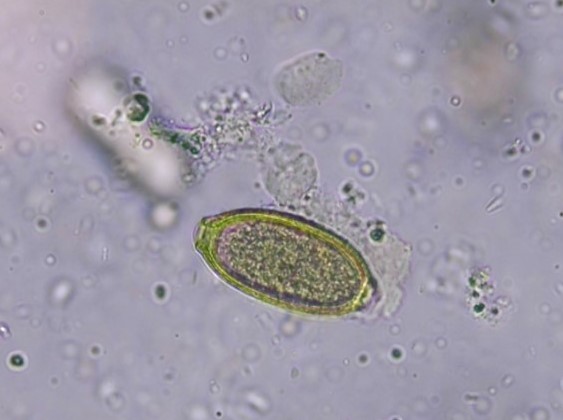
Figure 6: Egg of Capillaria, 40x magnification
Source: Laboklin
Prevalence
Lungworm infections in dogs and cats are uncommon, as we can also see in our own routine samples (Figure. 4). Some of these parasites are “emerging” (especially A. vasorum), some appear occasionally (e.g. C. aerophila) and others can be detected in Central Europe only sporadically (O. osleri, F. hirthi). Despite the apparent rarity, these parasites are of clinical relevance since they can cause severe disease with a possible fatal outcome.
Diagnosis
For the diagnosis, the presence of detectable parasites is necessary. It is of note, that during the prepatent period (the time between infection and the presence of detectable eggs or larvae), no diagnosis is possible.
Coproscopy
The microscopic detection of L1 larvae and eggs is the most common method. It is cost-effective, non-invasive and unspecific, and screening for different parasite species is possible. Despite the lower sensitivity of these tests, mainly due to intermittent shedding of lungworm larvae and Capillaria eggs, they should always be step one in any lungworm screening. To achieve the best results, collective faecal samples of three conse- cutive days should be examined. In the case of negative test results, repeat examinations are recommended, especially if there is a clinical suspicion for a lungworm infection.
Direct faecal smear
L1 larvae can be seen easily due to their active movement in fresh faecal samples. To prepare a faecal smear, a small amount of faeces is mixed with either water or saline solution on a glass slide, spread, covered with a cover slip and then examined microscopically. This method is very cheap and easy to perform, however, due to the very low amount of faeces used, the sensitivity is very low.
Flotation technique
For this method, a pea-sized faecal sample is suspended in a sugar- or salt solution with a known specific gravity. Faecal particles will precipitate, while the less dense parasites will float to the top, where they adhere to a glass cover slip and can be transferred to a glass side to be examined. This is the best way to detect Capillaria eggs, although other lungworm larvae could also be seen. Despite this, due to the limited amount of faeces used, the sensitivity is also limited.
Baermann-Wetzel technique for larval migration
A faecal sample of walnut-size is placed in a fine sieve or gauze, which is deposited in a funnel. The bottom of the funnel is connected to a rubber hose, which is tightly closed with a metal clamp. This apparatus is filled with lukewarm water, until the sample is covered half-way. Living larvae are attracted to moisture (hydrotropism). Over a period of 12-24h, they will migrate from the faecal sample and will accumulate at the bottom of the rubber hose. The first few water droplets from the hose will be transferred to a glass slide and examined microscopically (Figure 5).
This method is the gold-standard for the detection of lungworm larvae, however, there are limitations: it is relatively elaborate and takes a longer period of time. In addition, fresh faecal samples are needed, since only live larvae can migrate actively. The L1 larvae of O. osleri and F. hirthi are more lethargic and migration is slow and less efficient, therefore, they might only be detected with flotation, if at all.
Microscopy from further materials
While faecal samples are considered the best material in most cases, swabs or washes of the trachea as well as bronchoalveolar lavage (BAL) can also be examined microscopically for lungworm larvae. For parasites like O. osleri and F. hirthi, these samples are even more useful than faecal samples.
Identification of lungworm stages
The eggs of C. aerophila possess a structured eggshell, with a size ranging from 60 – 70 x 35 – 40 µm. They are brown, barrel-shaped and present with asymmetrical plugs at the egg poles (Figure 6). They have to be distinguished from the larger Trichuris eggs, which have a smooth shell and are lemon-shaped with symmetrical plugs at the poles. In addition, the eggs of other Capillaria species can be detected in the faeces, but all of these are very similar morphologically. Species differentiation is therefore not always possible.
L1 larvae of different metastrongylid lungworm species are also hard to distinguish morphologically. To differentiate these species, a meticulous examination, taking into account morphometry and morphology, is necessary. With appropriate experience, different species can be differentiated morphologically by worm length and shape of oral and caudal regions.
Lungworm larvae also have to be discerned from hookworm larvae (in older faecal samples) and if the samples have been taken from the ground, there is the possibility that free living or plant pathogenic nematodes may contaminate the sample. It is therefore important that faecal samples are collected as freshly as possible.
PCR testing
Specialized veterinary diagnostic laboratories offer specific PCR tests for the detection of different lungworm species. With this method, DNA of the parasites is amplified and visualized. A variety of sample materials can be used, including faeces, BAL, tracheal wash, deep throat swabs as well as lung tissue. For the detection of A. vasorum, EDTA whole blood can also be used. Although PCR in general is a very sensitive diagnostic method, s ensitivity might also be limited due to the biology of the parasites, e.g. intermittent shedding of larvae.
Antigen detection
For the detection of A. vasorum, there are several ELISA- and commercial rapid tests available. These are able to recognize freely circulating parasite antigens in serum samples.
Take home message
Lungworm infections should always be included as a differential diagnosis if dogs and cats are presented with cardio-pulmonal symptoms. Due to the intermittent shedding of worm larvae and l imited sensitivity of certain test methods, only positive results will be of clinical relevance and confirm the presence of these parasites. Fresh faecal samples are the most appropriate sample material.
Dr. Michaela Gentil
Service portfolio:
- Parasitological examination (sedimentation/ flotation)
- Baermann-Wetzel technique for larval migration
- PCR tests for Angiostrongylus vasorum, Crenosoma vulpis, Aelurostrongylus abstrusus and Troglostrongylus brevior
- Lungworm PCR profiles for dog and cat
- Angiostrongylus vasorum-antigen test