Acute-phase proteins (APP) are an important component of the body’s own innate immune system. The determination of individual APP is used in routine diagnostics to detect and monitor inflammatory reactions. However, it is important to be aware of species-specific differences.
APP are protein molecules which are produced in increased quantities – mediated by cytokines – when there is an inflammatory reaction. They are mainly produced by the liver. Their serum level already increases only a few hours after exposure to a noxious agent, often even prior to the onset of clinical signs or before changes can be seen in the blood count. They are therefore suitable biomarkers for inflammatory reactions. The level of APP often increases proportionally to the degree of inflammation. In turn, it rapidly decreases again after the noxious agent has been eliminated. APP are therefore also suitable for monitoring the course of an inflammatory reaction. The main noxious agents which trigger a reaction are:
- bacterial and viral infectious diseases
- aseptic inflammatory reactions
- autoimmune diseases
- traumata
- neoplasia
More than 30 different APP are known. Most APP belong to the fraction of alpha or beta globulins. Their concentration may not always be high enough to be reflected by electrophoresis. Furthermore, there may be an overlap with other protein components. It is therefore even more important to be able to routinely identify individual APP that are particularly conclusive. Depending on the extent of the increase, “positive APP” are categorised as follows: major APP, moderate APP and minor APP. In routine diagnostics, especially the major APP are important. Their serum level is very low in healthy animals, but they increase by 10- to 100-fold within a few hours of exposure to a noxious agent and decrease rapidly after the inflammation has subsided.
In contrast, a certain level of moderate or minor APP can also be detected when in good health, but they rise more slowly and not as much (up to 10-fold at most) and decline much more slowly. Thus, their significance in routine diagnostics is lower.
“Negative APPs” are another form. Their level decreases during an acute-phase response of the body. The best-known negative APP, which is regularly determined in routine diagnostics, is albumin. During an acute-phase response, the liver reduces albumin production by an average of about 10 to 30% in favour of the production of “positive APP”. Albumin can be used as a negative APP in all animal species.
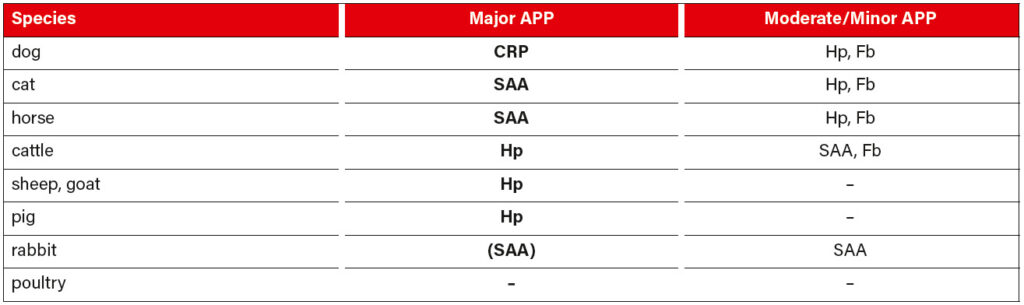
There are considerable species-specific differences in positive APP. Table 1 provides a brief overview of the APP that are currently most often used in routine diagnostics for the respective animal species.
Their functions vary between proteins and are very complex. The individual APP usually have several tasks, thus regulating the immune response. For example, they activate the complement cascade and facilitate phagocytosis and lysis of bacteria (CRP – C-reactive protein). Leukocytes can be chemotactically attracted and their adhesion in the inflammatory area can be promoted (SAA – serum amyloid A). Moreover, anti-inflammatory processes are triggered to counteract the inflammatory reaction (CRP, SAA). Free haemoglobin can be bound and transported to the liver for reuse. For one, it prevents iron loss, and secondly, it removes available iron resulting in a bacteriostatic effect (Hp – haptoglobin). The spread of the cause of the inflammation can be contained through the formation of a fibrin network (Fb – fibrinogen).
It is impossible to imagine routine diagnostics without APP. Even though they cannot give any information on the site or the cause of the inflammatory reaction, they are still of great use for many diagnostic tasks and are particularly suitable for
- the diagnosis of subclinical and chronic diseases
- the early detection of inflammatory reactions
- therapy monitoring
- monitoring the healing process
- monitoring post-operative convalescence.
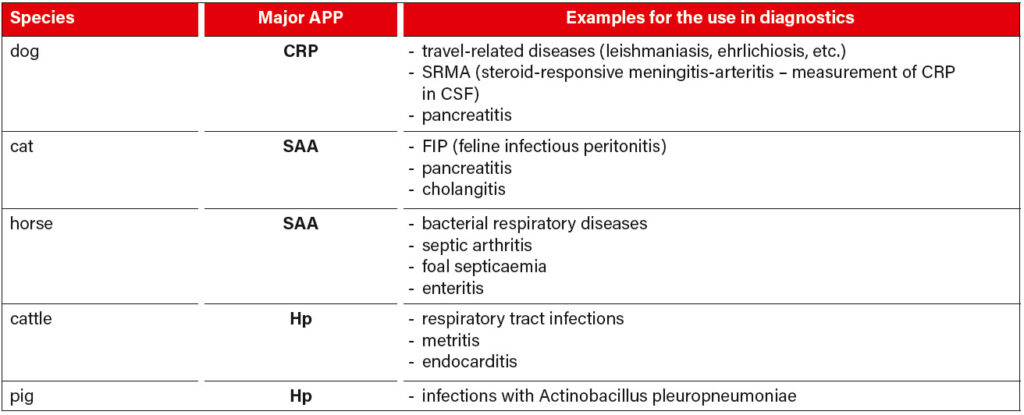
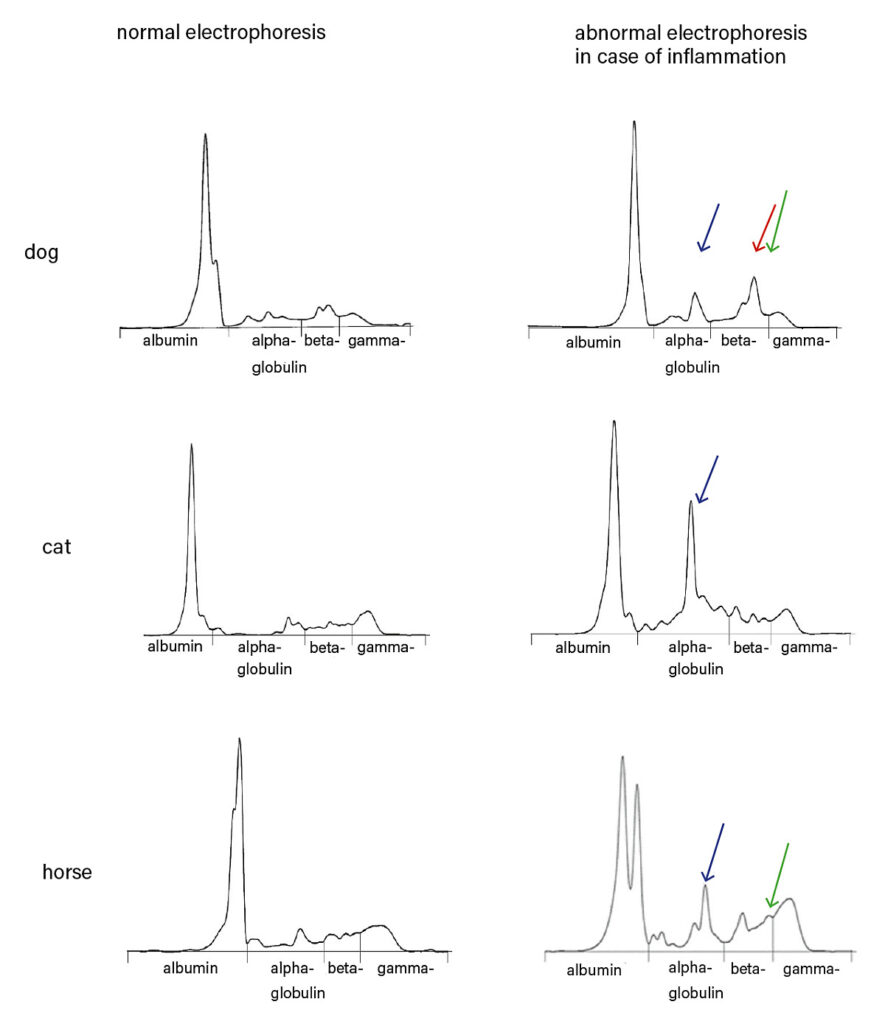
Table 2 shows some examples of the diagnostic use of APP in some species. If the clinical signs of a patient are unclear or if blood count and clinical chemistry are inconclusive, we recommend serum protein electrophoresis as an additional screening test. In most cases, the pattern indicates a direction for further examinations, which then lead to the diagnosis. APP also appear as typical peaks here, SAA and Hp in the alpha-2 fraction, CRP and Fb in the beta-2 fraction (for a comparison of electrophoresis patterns, see figure 3, page 4). However, due to an overlap in electrophoresis with other proteins with similar physical properties, the relevant APP should be measured quantitatively by clinical-chemical determination. It should be noted that plasma protein electrophoresis can show further peaks, as plasma still contains the coagulation factors. Especially fibrinogen can make it difficult to interpret the beta-2 fraction. It must also be borne in mind that both corticosteroids and NSAIDs can influence the clinical-chemical determination as well as the presence of APP in an electrophoresis run. This should always be taken into consideration when interpreting the results.
Dr. Ruth Klein, Dr. Karin Friedrich
-
Fig. 1: Diagnostic issue: inflammation? – APP (acute-phase proteins) help to make a diagnosis.
Source: Dr Ruth Klein
-
Tab. 1: APP that are being regularly used in routine diagnostics with reference to the individual species; CRP (C-reactive protein), SAA (serum amyloid A), Hp (haptoglobin), Fb (fibrinogen)
Source: Laboklin
-
Tab. 2: Examples for the use of major APP in the diagnosis of different species; CRP (C-reactive protein), SAA (serum amyloid A), Hp (haptoglobin)
Source: Laboklin
-
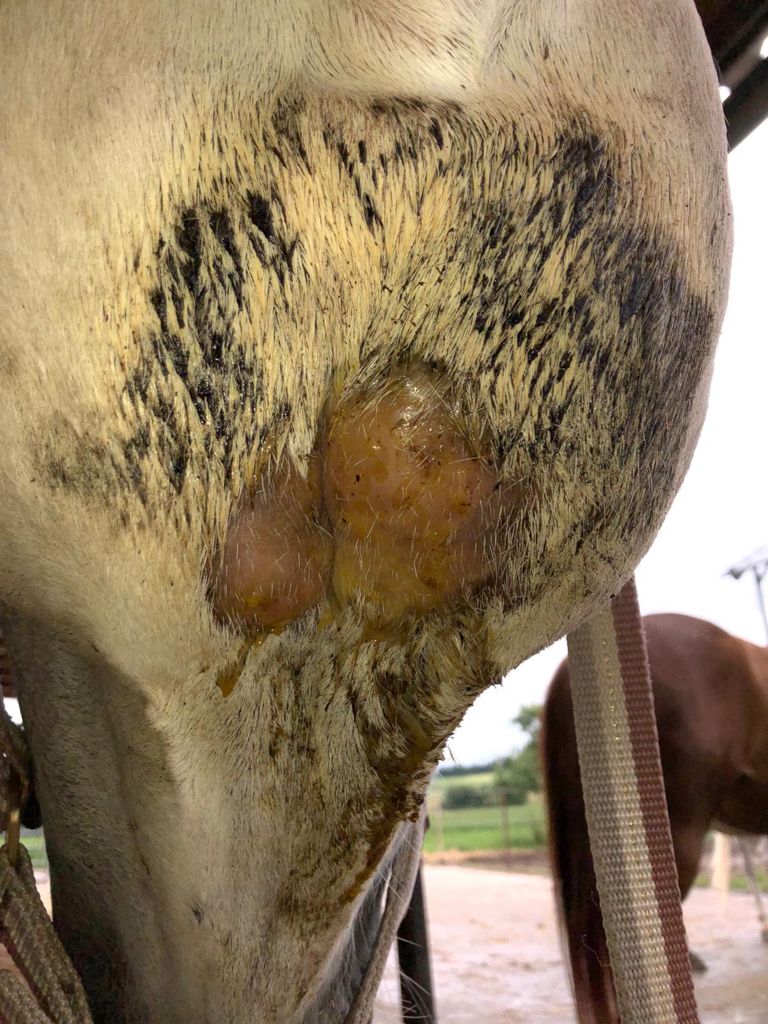
Fig. 2: Inflammatory enlarged mandibular lymph nodes with suspected abscess formation in a horse. Here, determination of acute-phase proteins (SAA) can be helpful for a more accurate assessment
Source: Laboklin
-
Fig. 3: Comparison of normal serum electrophoresis patterns with patterns indicating an inflammatory process. (blue arrow) indicates increased SAA and/or haptoglobin level; (red arrow) indicates increased CRP level; (green arrow) indicates high serum fibrinogen level and is common in plasma protein electrophoresis.
Source: Laboklin