In recent years, the number of domesticated alpacas and llamas as well as the absolute number of these animals has been steadily increasing. To date, there is no national register for New World camelids, and they are not included in the Animal Disease Notification System, the HI Animal Database (Animal Origin and Information System), or the Animal Disease Insurance Funds. New World camelids fall under the Animal Health Law and are subject to intra-community movement regulations. New World Camelids are susceptible to some of the animal diseases listed under the Animal Health Law and can potentially transmit these diseases to other animal species or to humans. It should be noted that infections with pathogens that are relevant under the animal health legislation may be subclinical in New World Camelids or may not present clinically in the same way as known in ruminants or horses.
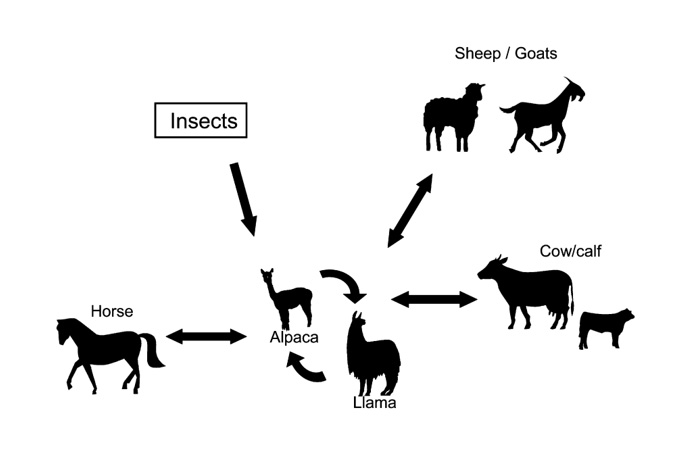
New World camelids can therefore act as both vectors or reservoirs, which is particularly important in countries and regions with sanitation and eradication programs. When New World Camelids are used for trekking, adventure holidays or animal assisted therapy, special consideration must also be paid to zoonotic pathogens (Fig. 1 and 2).
This section provides an overview of frequently requested animal disease tests and useful biosecurity tests for New World camelids.
When these animals are being traded, exported or are part of an exhibition certain pathogen and antibody tests are required by buyers, the competent veterinary authorities, or event organisers. At the same time, no tests have been specifically developed or validated for New World camelids. IFATs and ELISAs can potentially be carried out with serum from New World camelids, but the test manufacturers do not specify whether the interpretation of the results also applies to New World camelids. Traditional serological methods, such as CFT, RBT, or SNT, are also suitable for species-independent testing (Fig. 3). Additionally, direct pathogen detection is possible using PCR testing and bacterial culture. Depending on the question being asked and the possibility of obtaining suitable sample material, these methods should be preferred.
Fig. 3: Tests for pathogen and antibody detection
| ELISA | Enzyme Linked Immunosorbent Assay |
| IFAT | Indirect fluorescence antibody test |
| KBR | Complement binding reaction |
| RBT | Rose Bengal test |
| SNT | Serum neutralisation test |
| PCR | Polymerase chain reaction |
| BU | Cultivation |
In the context of trade and animal shows, the competent veterinary authorities often require proof that the animals are free from BHV-1, BTV, BVDV, SBV, brucellosis, and tuberculosis:
Bovine herpesvirus 1 (BHV-1):
BHV-1 infections in New World camelids are usually subclinical. In the clinical course, respiratory signs are prominent. Pathogen detection from a swab is possible using PCR. A serological test for antibodies against BHV-1 can be carried out independently of the animal species using SNT.
Bluetongue virus (BTV):
New World camelids are susceptible to BTV but usually present with fewer clinical signs compared to cattle and small ruminants. However, death with erosive and ulcerative oral mucosal defects and pneumonia have been described. The pathogen can be detected by PCR testing of EDTA blood or tissue. It should be noted that viraemia appears to be shorter in New World camelids than in ruminants. Antibodies have been detected in studies using ELISA, but the tests have not yet been validated for these animal species.
Bovine viral diarrhoea virus (BVDV):
New World camelids can become transiently (TI) and persistently (PI) infected with the BVD virus. Immunotolerant PI crias (= young alpaca or llama) develop in the womb when the pregnant mother is infected, although the exact period is not yet known. PI crias are noticeable as they show signs that they are failing to thrive. If antibodies against the BVD virus are detected in a herd, the search for the source of infection must include the identification of PI animals. PCR testing of EDTA blood, nasal swabs, or faeces is suitable for identifying PI animals. A serological test for antibodies against BVDV is possible regardless of the animal species using SNT.
-
Fig. 1: Alpaca or llama trekking involves close animal-human contact.
Image source: envatoelements
-
Fig. 2: Close animal-human contact at the petting zoo
Image source: Adobe Stock
-
Fig. 4: Alpacas and Llamas can be reservoirs and carriers of various animal diseases. They should not be kept together with ruminants or horses.
Image source: Kapil S, YearyT, Evermann JF: Viral Diseases of New World Camelids, Vet Lin Food Anim 25 (2009)
-
Fig. 5: Goats
Image sources: Adobe Stock
-
Fig. 6: When New World camelids are kept together with horses or ruminants, there is a possibility of transmission of infectious agents.
Image sources: Adobe Stock
-
Fig. 7:
Image sources: Adobe Stock
-
Fig. 8: Humans can play a role as vectors in the transmission of pathogens. At the same time, they must be protected from zoonotic infections.
Image sources: Adobe Stock
Schmallenberg virus (SBV):
New World camelids show a high seroprevalence against SBV, but with rather low titres. To date, there have been no reports of clinical symptoms, abortions, or malformed foetuses in connection with SBV infections. The pathogen can be detected by PCR testing of EDTA blood, placenta, or foetal tissue. Antibodies have been detected in studies using ELISA, but the tests have not yet been validated for these animal species.
Brucellosis:
New World camelids are susceptible to Brucella abortus and Brucella melitensis, and abortions can occur in infected animals. Serological testing for antibodies against Brucella spp. is possible regardless of species using RBT or CFT.
Tuberculosis (TB):
Testing for the Mycobacterium tuberculosis complex (MTC) is mandatory for intra-community trade in breeding animals. A laboratory diagnostic test for antibodies against MTC is not authorised in Germany, and the test can only be carried out on the animal using an intradermal test. It should be noted that false negative results occur frequently in New World camelids. The tuberculin is not registered for use in New World camelids, which means that the risk of use is transferred to the veterinarians (it is recommended that animal owners sign a corresponding information sheet). Due to the zoonotic potential and the limited testing options, monitoring is particularly important, and dead animals should be sent for necropsy.
Irrespective of any mandatory testing for animal pathogens, livestock farmers should take cognisance of the basics of biosecurity to protect their herds effectively from the introduction of infectious diseases. This means, among other things, recognising and minimising risks from the public and other animals, not keeping New World camelids together with ruminants or horses, and performing necropsies on dead animals (Fig. 4 to 8). Purchased animals should be clinically examined prior to transport, and samples should be taken for testing for relevant infectious diseases.
Depending on the epidemiology, quarantine and, if necessary, retesting on the new farm is recommended, even if the results are negative.
In addition to the required tests, there are other useful tests for maintaining the health of llama and alpaca herds:
Mycoplasma (M.) haemolamae:
This is a haemotropic intracellular bacterium that infects the erythrocytes of infected animals and can presumably be transmitted by blood-sucking insects and possibly also via other body fluids and potentially vertically within the herd. Infected animals do not necessarily become clinically ill but can develop severe haemolytic anaemia if challenged by illness or stress due to the pathogen multiplication. The examination for M. haemolamae is analysed by PCR from EDTA blood.
Pseudotuberculosis:
This chronic and untreatable disease is caused by Corynebacterium pseudotuberculosis, which can also infect sheep and goats. The pathogen encapsulates itself in lymph nodes and causes internal and/or externally visible or palpable abscesses. The pus is highly infectious.
A serological examination can be carried out using an ELISA test, which has been validated for New World camelids. It should be noted that due to the encapsulation of the pathogen, antibody formation may be low and/or delayed. Pathogen detection from abscess contents is possible via bacterial culture or PCR.
Paratuberculosis:
Alpacas and llamas are susceptible to Mycobacterium avium ssp. paratuberculosis (MAP). However, the prevalence in Central Europe appears to be low in New World camelids. Infected animals can excrete the pathogen for a long time and spread it within the herd without ever becoming clinically ill. Clinical disease with diarrhoea and emaciation is rare. Direct detection of the pathogen using PCR from faeces is recommended. Culture is also possible but extremely time-consuming. A suitable test for antibodies is currently not available for New World camelids, and existing ELISAs have not yet been validated for these species.
Parasitoses:
Gastrointestinal strongyles (MDS), particularly Haemonchus contortus, but also Trichostrongylus and the small liver fluke (Dicrocoelium dendriticum), have great potential for infecting New World camelids. Due to the widespread resistance of a large number of anthelmintics, an introduction into one’s own stock should be avoided as far as possible. Suitable diagnostics include a parasitological faecal examination and an egg count reduction test. This involves conducting a McMaster count before deworming and 10–14 days after deworming. Effective deworming reduces the number of eggs per gram of faeces by 95%. If the reduction rate is lower, it can be assumed that there is resistance to the active substances used.
Haemonchus differentiation via fluorescence staining is possible if MDS eggs are detected in the parasitological examination. For the large liver fluke (Fasciola hepatica), serological testing for antibodies using ELISA is possible.
Breeding:
Tests for infectious diseases relevant to breeding hygiene are important when acquiring breeding animals or when mating mares and stallions of different origins. A uterine or vaginal swab from the mare can be analysed by culture for fertility-relevant pathogens. Unfortunately, a swab or smear from the stallion is practically impossible to obtain due to the very poor accessibility of the penis.
A serological examination for antibodies can provide information on whether an animal has already had contact with certain pathogens. If an acute event is suspected, a paired serum sample is recommended at intervals of 2-3 weeks to determine a possible increase in titre.
Leptospirosis:
Infections with leptospira can cause febrile systemic diseases in New World camelids but can also lead to abortions in clinically normal mares.
The pathogen can be detected by PCR from the foetal kidney. Antibodies against leptospira can be analysed using MAT, and a paired serum sample is recommended.
Q-fever:
New World camelids are susceptible to Coxiella burnetii, and the pathogen is associated with abortions. Abortion material, uterine swabs, or milk, are suitable for direct detection using PCR.
ELISAs can be used for antibody detection; although these have not yet been validated for New World camelids, they have provided credible results in studies.
Toxoplasmosis and Neosporosis:
Infections of New World camelids with Toxoplasma (T.) gondii and Neospora (N.) caninum are possible and can cause abortion. Studies estimate the seroprevalence of T. gondii to be higher than that of N. caninum. It is assumed that infections with N. caninum poses a higher risk of abortion. Abortion material, uterine swabs, or foetal tissue (brain, heart) are suitable for pathogen detection by PCR. ELISA tests are available for serological testing and have produced good results in studies for the detection of antibodies. For toxoplasmosis, IgG and IgM are determined separately, allowing differentiation between an acute event and earlier contact with the pathogen.
Chlamydiosis:
Chlamydia spp. are also associated with abortions in New World camelids. PCR testing is possible using abortion material and uterine or vaginal swabs.
Antibody detection from serum can be performed using IFAT, and a paired serum sample is recommended.
Special camelid services
8176 Mycoplasma haemolamae
1183 Parasite profile
527 Haemonchus contortus
1252 Abortion profile serological
8431 Abortion profile PCR
Dr Anna-Linda Golob