Calcium
98% of the calcium (Ca) is contained in the bones and ensures their stability. In addition, it is also important for blood coagulation and muscle contraction. It is ingested with the food: bones, bone meal and egg shells contain calcium. Calcium is excreted in urine, faeces and, to a small extent, in sweat. The calcium and phosphorus balance is regulated by absorption from the small intestine, incorporation in or demineralisation of the bones and renal excretion. Parathyroid hormone (PTH), which is produced in the parathyroid gland, plays a central role here.
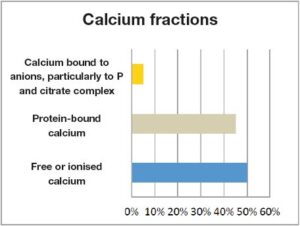
Despite the influence of many exogenous factors, such as varying mineral supply, this regulatory system can maintain homeostasis for a long time. Especially calcium is regulated very tightly. This means that a calcium level which is not within the normal range should always be verified, even if it deviates only slightly. Determination of calcium in the blood: Calcium consists of 3 fractions: 50% as free or ionised calcium, 45% protein-bound calcium, 5% calcium bound to anions, particularly to P and citrate complex (Fig. 1).
Protein-bound calcium (routine diagnostics)
In serum, protein-bound calcium is often measured as it can be determined more easily than ionised calcium because it is less influenced by pre-analytics.
However, its concentration in serum is influenced by the total protein, especially albumin. A drop in albumin levels causes a decrease in serum calcium.
Ionised calcium
This is a better indicator of the biologically active calcium because serum or plasma concentrations are directly controlled by PTH and calcitriol. It is therefore the more sensitive method for measuring disturbances of calcium balance.
However, pre-analysis is much more complex. Ionised calcium can only be determined if collected under exclusion of air (sampling instructions are available from Laboklin).
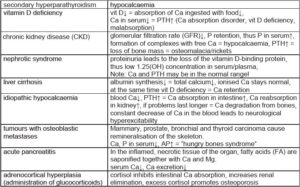
Examples of hypocalcaemia
(Tab. 1)
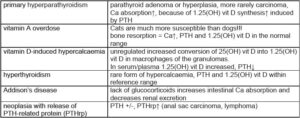
Examples of hypercalcaemia
(Tab. 2)
- Fig. 1: Calcium fractions
- Tab. 1: Examples of hypocalcaemia
- Tab. 2: Examples of hypercalcaemia
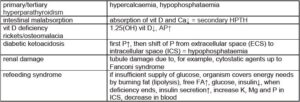
- Tab. 3: Examples of hypophosphataemia
- Tab. 4: Examples of hyperphosphataemia (physiological in young animals)
- Tab. 5: Examples of hypomagnesaemia
Inorganic phosphorus (P)
The terms phosphorus and phosphate are used interchangeably in laboratory medicine. For clinical purposes, this is irrelevant because the phosphate content is measured as inorganic phosphorus. 85% of the phosphate is located in the bones in combination with calcium and 14% exists intracellularly. There, it is present as an anion or as a component of lipids, proteins and nucleic acids. 1% of the P content is located in plasma or in other body fluids, yet in most pathological conditions, the concentration in serum correlates with the phosphate content of the body. Renal reabsorption is an important indicator of the phosphate level in the serum. If phosphate absorption is increased or GFR is decreased, renal reabsorption is decreased. Renal reabsorption is regulated by fibroblast growth factor (FGF) and parathyroid hormone.
Examples of hypophosphataemia
(Tab. 3)
Hyperphosphataemia
Hyperphosphataemia lowers the concentration of 1.25(OH) vit D and increases the secretion of PTH and FGF. These hormones have a phosphaturic effet.
Examples of hyperphosphataemia (physiological in young animals)
(Tab. 4)
Magnesium
1% of the magnesium (Mg) is found in the extracellular fluid. The Mg fractions in serum and plasma are present as ionised magnesium, protein-bound magnesium, mostly to albumin, and complex-bound magnesium in form of salt. The skeletal system, the gastrointestinal tract and the kidneys regulate the magnesium concentration in the plasma. Magnesium depends on the albumin concentration and the pH value. If there is alkalosis, the Mg level is reduced by increased protein binding. However, a reduction may also be seen with normal serum levels. Magnesium has many different tasks: It is essential for electrolyte balance, energy metabolism (activation of ATP), neural conduction, protein synthesis, bone matrix formation and mineralisation of the skeleton as well as for cell division.
Examples of hypomagnesaemia
(Tab. 5)
Hypermagnesaemia
Except for severe CRI, hypermagnesaemia is unlikely to occur with adequate substitution.
Serum electrolytes and nutrition
When evaluating serum electrolytes, special attention should be paid to nutrition. Calcium is only ingested with the food through bones, bone meal and egg shells. Muscles and innards, vegetables and fruit are significantly lower in calcium. Ca deficiency in the diet results in increased PTH secretion. This leads to mobilisation of Ca from the bones and increased absorption from the intestine. In the long term, bone demineralisation develops and even bone deformation, particularly of the long bones, as well as other changes in the skeletal system. A typical sign is the so-called “rubber jaw”. Ca oversupply, on the other hand, which occurs much less frequently for nutritional reasons, leads to tissue calcification, stomach ulcers and impaired muscle contraction up to tetany. Severely disturbed bone metabolism, especially in the long bones, and increased urinary excretion of calcium are signs of Ca oversupply. Phosphate is present in many food products (meat, vegetables and fat). This means that phosphorus intake from food is usually sufficient. Phosphate is stored in the skeletal system and regulated by the kidneys. Food should have a Ca/P ratio of 2:1 or maybe 1.5:1. Inadequate supply, even inverse Ca/P ratios, are often seen in individual rations. Magnesium is mainly found in green vegetables, nuts, cereals, seafood and meat. But also drinking water, especially hard water, contains magnesium.
Conclusion
Concerning serum levels and nutrition, it is particularly important to note that serum levels in the normal range do not necessarily reflect a balanced diet. Serum electrolyte levels in the reference range do not imply that the ration sufficiently meets the needs for these parameters. A balanced ration calculation is required instead.
Dr. Anja Cölfen