General information
The parameters we use to assess kidney function are so-called biomarkers. If the kidney does not work as it should, they remain in the blood. By looking at their levels, we can deduce the severity of renal dysfunction. Sounds simple, and it actually is.
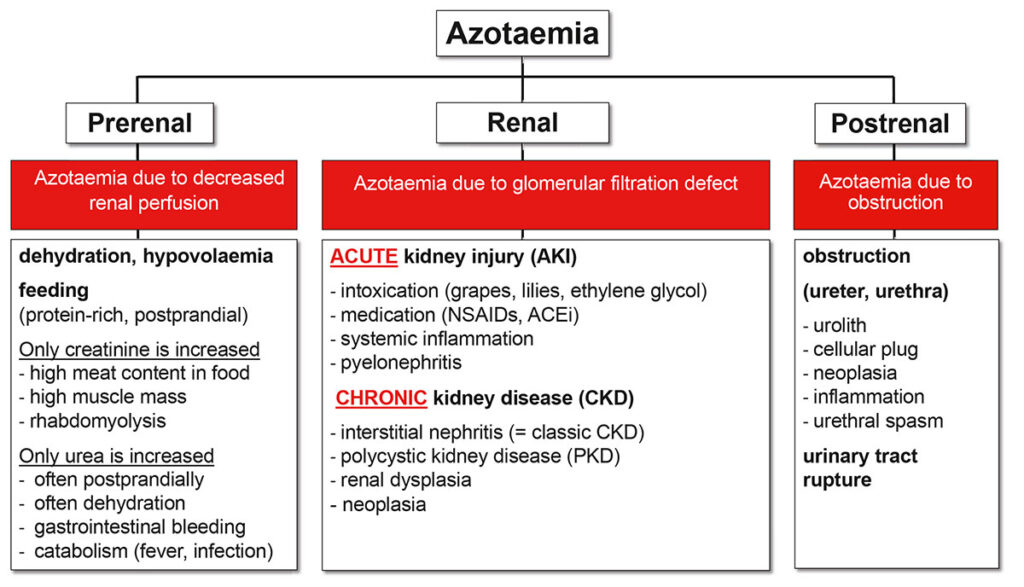
Azotaemia: The increase of urinary excreted substances in the blood is called azotaemia. The typical biomarkers are urea and creatinine. However, the presence of azotaemia does not automatically mean that the kidney is affected. Only once we are sure that there is neither prerenal nor postrenal azotaemia, we call it kidney disease.
Uraemia: Uraemia is a term used to describe the clinical consequences of renal dysfunction resulting from retention of toxic metabolites, dysregulation of water and electrolyte balance as well as hormonal imbalances. Signs associated with uraemia include lethargy, weakness, dehydration, inappetence, vomitus and weight loss.
Thus, azotaemia and uraemia are not the same. Renal azotaemia indicates renal dysfunction.
Uraemia signifies that the patient’s quality of life is affected. The former is therefore important for the diagnosis of kidney disease, the latter for assessing the clinically relevant severity of the disease. The difference between azotaemia and uraemia explains, among other things, why some patients with chronic kidney disease (CKD) still have a rather good general condition even with high kidney values, while others with lower values already feel worse.
The traditional biomarkers may be indicators of the degree of renal dysfunction, but do not necessarily indicate what impact this has on the individual patient. For most uraemic toxins that make our patients’ lives difficult, there are no commercially available test methods.
An exception to this is indoxyl sulphate (see below).
-
Fig. 1: Differential diagnoses for azotaemia
Source: Dr. Jennifer von Luckner
-
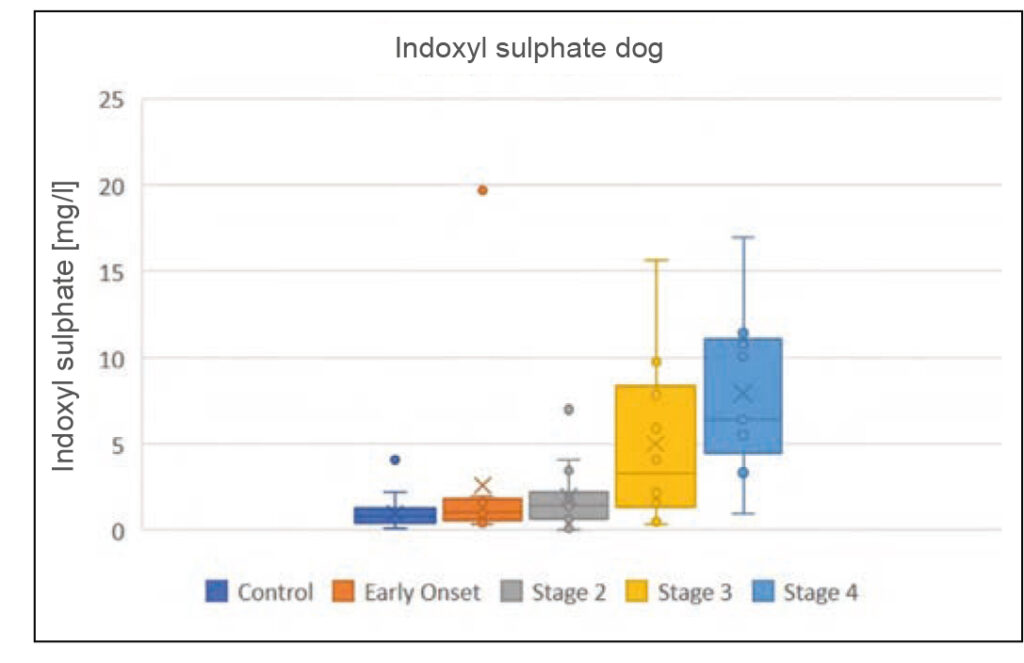
Fig. 2a: Indoxyl sulphate concentrations in dogs and cats with CKD, depending on the IRIS stage
Source: Laboklin, unpublished data
-
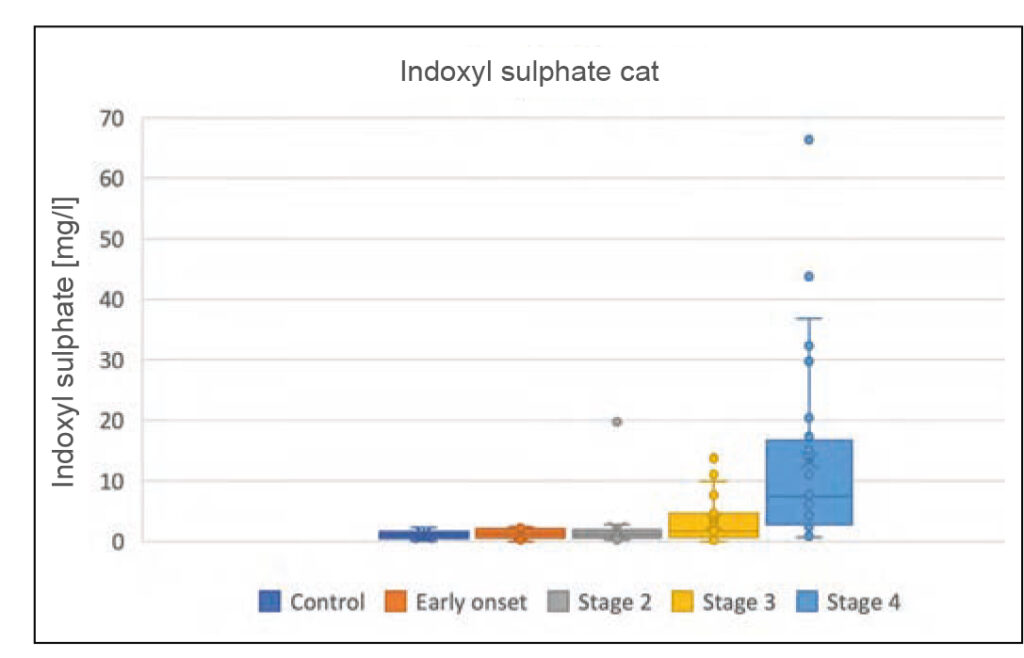
Fig. 2b: Indoxyl sulphate concentrations in dogs and cats with CKD, depending on the IRIS stage
Source: Laboklin, unpublished data
In the kidney, the blood passes through the glomerulus to be cleaned. There, the ultrafiltrate is pressed out (glomerular filtration). This ultrafiltrate is further processed in the tubule, where the substances the body does not want to lose (e. g. water, proteins, glucose, electrolytes) are reabsorbed.
The biomarkers determined in the traditional kidney profile (especially creatinine and SDMA) mostly reflect the glomerular filtration rate (GFR). However, reduced GFR alone does not define renal dysfunction. Tubular processes play an essential role. It is these processes that lead to typical problems such as polyuria/polydipsia, dehydration and electrolyte imbalance in animals with kidney disease. Furthermore, many uraemic toxins are not only excreted into the urine by glomerular filtration, but must additionally – or sometimes even mostly– be secreted through the tubule. Especially in advanced stages of interstitial nephritis, the most common cause of chronic kidney disease in dogs and cats, tubular function becomes increasingly poor and thus clinically relevant for us.
Biomarkers for kidney function tests
Urea
Urea is a waste product of protein metabolism. It is filtered freely through the glomerulus, but partially reabsorbed in the tubule. Urea is therefore not a precise marker of GFR. During diuresis, urea in the blood decreases due to reduced reabsorption. In case of dehydration or other perfusion disorders, its increase in concentration is stronger than that of the other renal parameters. Furthermore, the blood urea level is considerably influenced by the amount of protein ingested with the food (increase with high-protein feeding, decrease in inappetent animals).
Gastrointestinal bleeding also leads to increased concentrations of urea. This makes urea a rather difficult parameter when it comes to assessing GFR. Yet, there is one advantage of urea: Even though it is not toxic itself, an elevated level often correlates well with uraemia.
Ideally, urea is determined from serum. The blood should be centrifuged and pipetted. Otherwise, incorrectly high results may be measured due to possible haemolysis during transport. Apart from haemolysis, hyperbilirubinaemia can also lead to increased urea concentrations. Lipaemic blood may reduce the measured value. The terms UREA and BUN (blood urea nitrogen) often cause confusion.
These parameters are not exactly equivalent. Only UREA is measured directly. BUN is determined via an indirect measurement method, which does not detect the entire urea molecule, but only the nitrogen contained in it. This is typical for in-house devices. If both values (UREA and BUN) should be compared, correction formulas need to be used.
Creatinine
Creatinine is produced by muscle metabolism and thus depends on muscle mass. It is filtered through the glomerulus but not reabsorbed in the tubule. It therefore correlates very well with GFR. Contrary to widespread belief, creatinine concentrations begin to increase relatively early in kidney disease, but only if GFR is reduced by 70 – 85%, creatinine rises above the reference range (=creatinine-blind range). It is advisable to take a closer look at this value in the kidney profile. If it is higher than would be expected for the patient (e. g. in a patient with little muscle mass) or if it increases over time, it can be a first indication of renal dysfunction.
Although creatinine is generally a good parameter for assessing GFR, it should be noted that it is not always increased in proportion to the stage of renal disease. In old animals with little muscle mass as well as in cases of hyperfiltration due to systemic hypertension or in hyperthyroidism, creatinine may be lower than renal function would actually suggest.
Apart from renal disease, elevated levels are seen in dehydration and perfusion disorders (prerenal azotaemia), urinary tract obstruction/rupture (postrenal azotaemia), in very well-muscled and trained dogs as well as in Birman cats and when feeding a diet rich in meat.
The IRIS (International Renal Interest Society) mainly focuses on creatinine to classify kidney disease. The classification of CKD into stages 1 – 4 is particularly relevant in everyday practice. In stage 1 (early stage), there is no azotaemia. Stage 2 is defined by a low degree of azotaemia, where there are often no or only mild clinical signs. Stage 3 is characterised by moderate azotaemia and more obvious clinical problems. Stage 4 is often referred to as the final stage; azotaemia is severe.
SDMA
Symmetric dimethylarginine (SDMA) is an amino acid derivative that is released during protein degradation. SDMA is filtered through the glomerulus and is pratically not reabsorbed. This makes it another parameter for GFR. The advantage over creatinine is that the SDMA concentration is less dependent on muscle mass and SDMA is therefore also diagnostically conclusive in cachectic animals. SDMA can be used for the early detection of kidney disease. Concentrations can be increased even if there is only a very mild decrease in GFR.
However, this is not constantly the case. Within the biological variability, SDMA may even increase later than creatinine in some patients. It should be kept in mind that SDMA, just like creatinine, is influenced by factors that affect GFR regardless of the health status of the kidney. It increases as a result of dehydration and reduced renal perfusion (prerenal azotaemia) as well as in cases of urinary obstruction (postrenal azotaemia) and decreases when there is hyperfiltration (hyperthyroidism, systemic hypertension). It is often elevated in growing animals and those with increased protein turnover. In general, a single increase in SDMA concentration should be checked several times if the renal profile is otherwise normal. Only if there is a permanent elevation, it indicates kidney disease.
The IRIS included SDMA in its staging of chronic kidney disease.
|
IRIS Stages |
Creatinine [μmol/l] |
SDMA [μmol/l] |
||
| Dog | Cat | Dog | Cat | |
| 1 | < 125 | < 140 | < 0.89 | < 0.89 |
| 2 | 125 – 250 | 140 – 250 | 0.89 – 1.73 | 0.89 – 1.24 |
| 3 | 251 – 440 | 251 – 440 | 1.78 – 2.67 | 1.29 – 1.88 |
| 4 | > 440 | > 440 | > 2.67 | > 1.88 |
Tab. 1: IRIS staging of CKD, adapted to the SDMA test used at Laboklin (SI units) Source: Laboklin
Cystatin C
Cystatin C is used for an early diagnosis in humans, and some authors consider it to be superior to the determination of creatinine in dogs. However, a recent study found the sensitivity and specificity of cystatin C to be lower compared to creatinine and SDMA. Moreover, care should be taken if dogs suffer from D. mellitus or hyperadrenocorticism. In feline CKD, a fairly strong overlap in values was found between healthy and affected cats. Consequently, cystatin C is an unreliable parameter in cats.
Indoxyl sulphate
Indoxyl sulphate is one of the most important uraemic toxins. It is a degradation product of indole produced during tryptophan metabolism. 90% of the protein-bound toxin is secreted into the urine by tubular transporters and 10% is filtered through the glomerulus. If it is not adequately excreted due to a combined malfunction of the glomerulus and the tubule, it induces oxidative stress, which leads to further damage and thus to the progression of kidney disease. It is also likely to have a negative effect on phosphate balance, which is important for renal progression. Indoxyl sulphate correlates with GFR and serum urea, creatinine and phosphate concentrations in dogs and cats with kidney disease. It already increases slightly in early stages of the disease (IRIS stage 2) and is highest in animals with advanced renal dysfunction (IRIS stage 4).
Since indoxyl sulphate is a uraemic toxin, it does not only indicate the degree of GFR reduction, but, instead, also provides information about the clinically relevant condition of the patient.
Thus, indoxyl sulphate can be a practical help for therapeutic decision-making as well as for assessing the effectiveness of therapeutic measures carried out. Furthermore, it seems promising as a valuable prognostic marker. The higher the value, the more it is necessary to work on therapeutic measures to reduce uraemic toxins. Blood should be collected when the animal is fasted, as indoxyl sulphate increases after high-protein feeding. This test requires serum (centrifuged + pipetted) which must be sent refrigerated. Determination of the parameter is done using the rather complex HPLC method and is currently only offered by Laboklin.
Phosphate
Phosphate is excreted by the kidneys and is therefore also a marker of impaired renal function.
However, patients in earlier stages of CKD (IRIS 1 + 2) usually have phosphate concentrations within the reference range due to a counter-regulation which is initiated when phosphate is retained. This counter-regulation is mainly controlled by parathyroid hormone and leads to a reduced reabsorption of phosphate in the tubule. As a result, the body can keep the blood phosphate level in balance. Yet, this only works if there is enough time to activate this compensatory mechanism and if the amount of reabsorbed phosphate does not exceed the amount that can be compensated. In later stages of CKD (usually from IRIS stage 3 on), the reduced filtration of phosphate can no longer be compensated by a lower reabsorption rate in the tubule. It is not until then that phosphate levels rise above the reference range in CKD. It should be noted that not only the phosphate itself, but mostly the compensatory mechanisms are considered responsible for the progression of kidney disease.
It is therefore recommended to keep the phosphate level below a certain target range. This target range is different than the reference value. For example, IRIS recommends a phosphate level between 0.9 and 1.5 mmol/l (2.7 – 4.6 mg/dl) for dogs and cats with CKD (especially in early IRIS stages). The recommended maximum limit for phosphate concentrations is lower than the upper reference value which is usually used.
Remember to do a urinalysis
A simple but often underestimated parameter for renal function is urine-specific gravity (USG). It is determined with a refractometer. The USG value which can be read on some urine test strips does not yield correct results for animals. USG is a parameter used to assess tubular function. If the ability of the kidney to concentrate urine is impaired, it indicates that the tubule does not sufficiently fulfil its function of reabsorbing water. Lower USG values may already be seen before the onset of azotaemia.
Typical values for kidney disease range from 1008 to 1025 in dogs and from 1008 to 1035 in cats.
However, other factors that can lead to decreased USG (e. g. bacterial infections, hormonal disorders, hypercalcaemia) must be excluded.
Proteinuria can also indicate kidney disease. Similar to decreased USG, this is already possible before the onset of azotaemia. It should be borne in mind that the urine test strip for cats tends to show both falsely increased as well as falsely decreased values. For this species, a reliable result can only be achieved by means of the urine protein-creatinine ratio (UP/C). UP/C should therefore be determined in cats even if the urine test strip does not show any protein. In dogs, determination of the UP/C is recommended to correctly quantify proteinuria if the urine test strip yields a positive result or if USG is very low (< 1012). Proteinuria is only indicative of kidney disease if there are no signs of inflammation in the urine. It is therefore advisable to always perform a sediment analysis at the same time. Other factors that can lead to (non-renal) proteinuria mainly include: systemic hypertension, hyperthyroidism, hyperadrenocorticism, fever and hyperproteinaemia. For follow-up examinations, it should be kept in mind that decreasing UP/C can only be considered an improvement if the kidney function is stable. If the kidney function deteriorates (indicated by increasing blood creatinine concentrations), there are fewer glomeruli available through which protein can be lost. UP/C may decrease even though kidney disease is progressive.
Conclusion
The standard kidney profile consists of biomarkers that primarily reflect GFR. SDMA is suitable for the early detection of kidney disease.
The importance of urinalysis should not be underestimated. USG and UP/C ratio can indicate renal dysfunction very early.
In later stages of CKD, urea and particularly indoxyl sulphate can help to assess the consequences of the disease for the patient.
Dr. Jennifer von Luckner, Dr. Corinna Weber
The list of references is available on request.