Physiology
Cortisol, which is produced in the adrenal glands, is absolutely essential in physiological concentrations. Cortisol regulates the metabolism by increasing glyconeogenesis, increases the reactivity of blood vessels, influences osmoregulation and electrolyte balance and has anti-inflammatory properties.
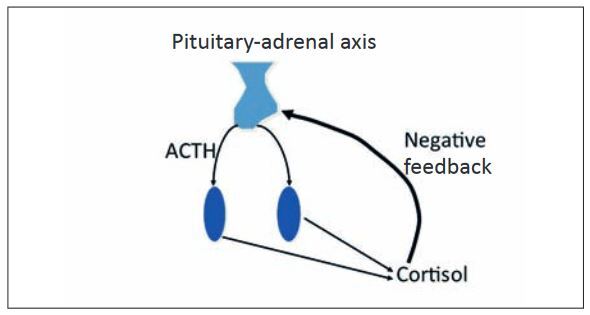
In order to ensure that the body does not produce too much cortisol, its production is regulated by the hypothalamic-pituitary-adrenal axis. The hypothalamus secretes CRH (corticotropinreleasing hormone), which stimulates the hypothalamus to secrete ACTH (adrenocorticotropic hormone). ACTH causes an increase in corticol secretion in the adrenal glands. The hypothalamic-pituitary-adrenal axis is controlled by a negative feedback loop, which prevents the production of too much cortisol. Cortisol therefore inhibits the secretion of CRH and ACTH.
Pathophysiology
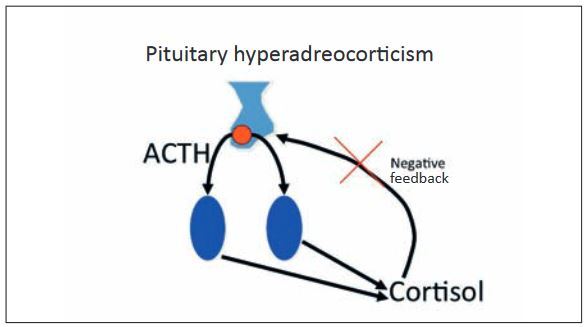
Overproduction of cortisol leads to hyperadrenocorticism, which is also known as Cushing’s syndrome. It is primarily caused by pituitary tumours (80-85%), whereby micro (85%) or macroadenomas (15%) secrete ACTH uncontrollably, which then leads to cortisol secretion in the adrenal glands. In these animals, cortisol levels are increased and ACTH is normal or increased:
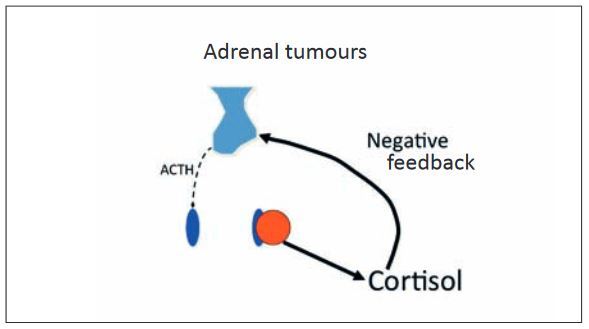
The remaining 15-20% are generally caused by unilateral benign or malignant adrenal tumours, which uncontrollably secrete cortisol. In these animals, cortisol levels are increased, while ACTH is reduced.
Dogs with pituitary hyperadrenocorticism are 2-16 years old (average 7-9) and can be any sex or breed, although smaller dogs and poodles, dachshunds, and terriers (Yorkshire, Jack Russell, Staffordshire, Bull) are most commonly affected.
Dogs with adrenal hyperadrenocorticism are older: 6-16 years old (11-12). Large breeds (>20 kg) are most often affected.
The typical clinical signs in both cases are caused by increased cortisol levels in the blood. The same clinical signs can also be found in dogs that havebeen treated with exogenous steroids.
Diagnosis
Cushing’s syndrom is a clinical diagnosis in dogs. The following clinical signs are observed in dogs with hyperadrenocorticism (Peterson, 300 dogs):
• Polyuria/polydipsia 82%
• Pot belly 67%
• Hepatomegaly 67%
• Alopecia 63%
• Lethargy 62%
• Polyphagia 57%
• Muscle weakness 57%
• Anoestrus 54%
• Obesity 47%
• Muscular atrophy 35%
• Comedones 34%
• Panting 31%
• Testicular atrophy 29%
• Hyperpigmentation 23%
• Calcinosis cutis (pathognomonic) 8%
• Facial paresis 7%
Unspecific tests should be carried out at first if Cushing’s syndrome is suspected in a patient. Blood chemistry, haematology, and urinalysis are helpful. The following results are expected (Peterson, 300 dogs):
• ALP (AP)↑ 86%
• Eosinopenia 84%
• ALT↑ 53%
• Hypercholesterinaemia 48%
• Hypophosphataemia 38%
• Total CO2↑ 33%
• Leukocytosis 32%
• Erythrocytosis 17%
• Lymphopenia 14%
- Fig. 1: Pituitary-adrenal axis
- Fig. 2: Pituitary hyperadreocorticism
- Fig. 3: Adrenal tumours
- Tab. 1: Low-dose dexamethasone suppression test
- Tab. 2: ACTH stimulation test
- Tab. 3: Urine Cortisol/Creatinine-ratio (diagnostic)
- Tab. 4: Endogenous ACTH
- Tab. 5: High-dose dexamethasone suppression test
Almost all dogs also have a decreased urine specific gravity and recurring urinary tract infections. If hyperadrenocorticism is supported by clinical findings, the diagnosis must be confirmed or ruled out using specific tests. The various tests available are discussed below.
Diagnostic Tests
• Urine Cortisol/Creatinine-ratio (screening)
1. Collection of morning urine.
This test has a very high sensitivity (95%), i.e. if the ratio is not increased, you can be very sure that the dog is not suffering from hyperadrenocorticism. The test’s specificity, however, is low; up to 76% of all positive animals do not have Cushing’s syndrome. This test is recommended when it is unlikely that the animal has Cushing and you are trying to rule out this diagnosis. If the test is positive, another test is necessary for confirmation.
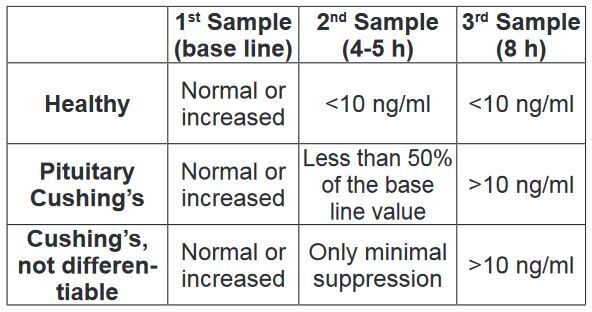
• Low-dose dexamethasone suppression test
1. First blood collection, baseline value.
2. Administration of dexamethasone at 0.01-0.02 mg/kg i.v. or i.m.
3. Second blood collection 4-5 h after administration of dexamethasone.
4. Third blood collection 8 h after dexamethasone administration.
This test also has a very high sensitivity (95%), i.e. if the result is negative, it is very improbable that the dog has Cushing’s syndrome. False negative results are primarily caused by inaccuracies in the calculation of the dexamethasone dose. It is important to note that the dexamethasone must be diluted before application in small dogs. Another advantage of this test is that it can differentiate between pituitary and adrenal hyperadrenocorticism in about 60% of the positive cases.
In order to keep the number of false positive results as low as possible, the consensus statement “Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal)” (J Vet Intern Med 2013; 27: 1292-1304) recommends only testing animals with at least three of the clinical signs described above and an increased alkaline phosphatase. If this is done, i.e. only those animals are tested in which Cushing’s syndrome is likely, this test is considered the “gold-standard” for the diagnosis of Cushing’s syndrome. The lowdose dexamethasone suppression test cannot be used to diagnose an iatrogenic Cushing’s syndrome. (Tab. 1)
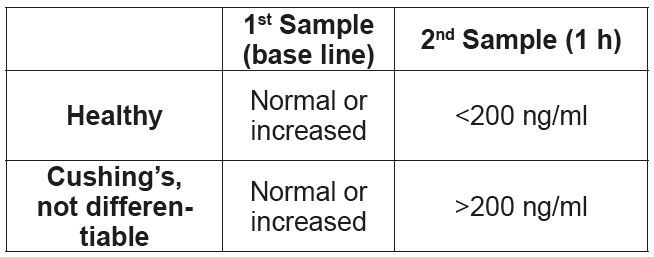
• ACTH stimulation test
1. First blood collection, base line value.
2. Injection of 5 μg/kg ACTH (Synacthen®) i.v. (alternatively, the entire vial (0.25 mg) can be injected i.v. or i.m.). Leftover Synacthen® can be kept frozen in a plastic container (e.g. a syringe) for 6 months.
3. Second blood collection one hour after administration of ACTH.
The advantage of the ACTH stimulation test is that it only takes one hour. It is also the test of choice for suspected iatrogenic Cushing’s syndrome or as a therapy control. The specificity of the ACTH stimulation test is comparable to the low-dose dexamethasone suppression test, i.e. it is important to prescreen animals before testing. The greatest disadvantage compared to the low-dose dexamethasone suppression test is the much lower sensitivity (80%) – false negative results are therefore relatively common and a negative result, in contrast to the other described tests, cannot be used to rule out the disease. The ACTH stimulation test also cannot be used to differentiate a pituitary from an adrenal hyperadrenocorticism. (Tab. 2)
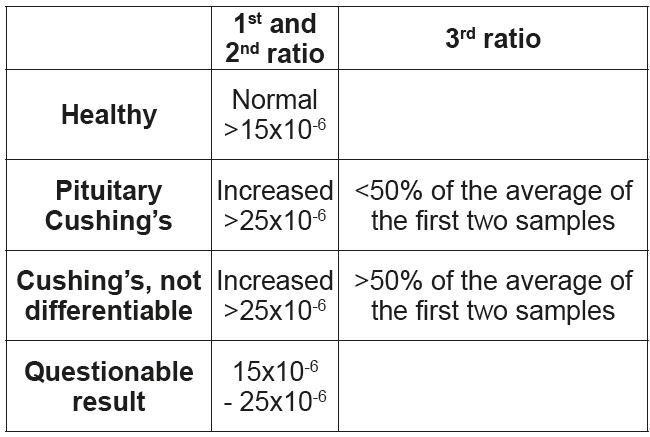
• Urine Cortisol/Creatinine-ratio (diagnostic)
1. Collection of morning urine on day 1 (first sample).
2. Collection of morning urine on day 2 (second sample).
3. Oral administration of dexamethasone on day 2: 3 x 0.1 mg/kg, over the course of the day.
4. Collection of morning urine on day 3 (third sample)
This test has the highest sensitivity compared to the other tests, but a low specificity. The first and second samples are used to diagnose hyperadrenocorticism, the third ratio can be used to differentiate between pituitary and adrenal Cushing’s syndrome in some dogs. (Tab. 3)
Tests for Differentiation
Once a diagnosis of Cushing’s syndrome has been made, the following tests can help differentiate between pituitary and adrenal hyperadrenocorticism.
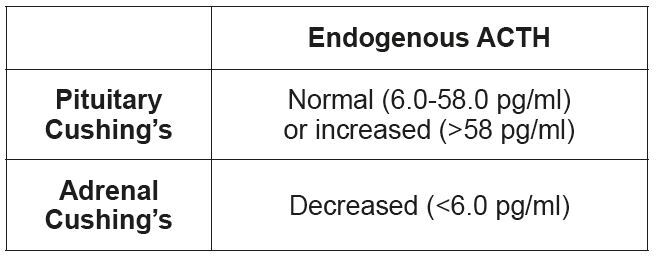
• Endogenous ACTH
Measuring the endogenous ACTH is used only for differentiation and cannot be used for diagnosis. In dogs with pituitary hyperadrenocorticism, endogenous ACTH is increased; in dogs with adrenal hyperadrenocorticism it is either decreased or normal.
Following sample collection into a plastic tube (ACTH binds to glass) with EDTA, the sample must be immediately centrifuged before the plasma is sent cooled to the laboratory. (Tab. 4)
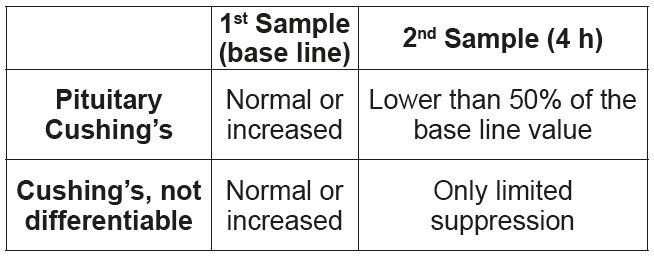
• High-dose dexamethasone suppression test.
1. First blood collection, base line value.
2. Administration of 0.1 mg/kg dexamethasone i.v. or i.m.
3. Second blood collection 4 h after administration of dexamethasone.
A differentiation between pituitary and adrenal hyperadrenocorticism is possible, if the cortisol concentration in the second sample is at least 50% lower than the base line value. If the value is suppressed by less than 50%, no differentiation is possible. (Tab. 5)
• Adrenal ultrasound
In cases of pituitary hyperadrenocorticism, both adrenal glands are enlarged, in adrenal hyperadrenocorticism, one adrenal gland is enlarged (by a tumour), while the other is atrophied.
Special cases
• Diabetes and Cushing’s syndrome
Cushing’s syndrome can cause poor glycaemic control. Diagnosis in these cases can be very difficult. Treatment of the diabetes before testing for Cushing’s syndrome once at least moderate glycaemic control has been achieved is recommended. The preferred test is a low-dose dexamethasone suppression test. An uncontrolled diabetes mellitus is the most common cause of false positive low-dose dexamethasone suppression tests. In these cases, it is particularly important to look at the clinical signs associated with the disease.
• Cushing’s syndrome in epileptic dogs treated with phenobarbital
Chronic administration of phenobarbital can cause the same clinical signs and laboratory results as Cushing’s syndrome. It is therefore recommended that phenobarbital be discontinued for at least six weeks before a dog is tested for Cushing’s syndrome (Canine & Feline Endocrinology, Feldman and Nelson, 4th Edition). Imepitoin and potasium bromide can still be given.
• Atypical Cushing’s syndrome
If a dog shows clinical signs typical for Cushing’s syndrome, but all specific tests are negative, an ACTH stimulation test should be carried out and 17-α-hydroprogesterone should be measured. In positive cases, treatment with trilostane (Vetoryl ®) should be considered. False positive results are possible.
Therapie
The medication that is approved for use in Europe is trilostane (Vetoryl®). It reversibly inhibits hydroxysteroid dehydrogenase, one of the main enzymes of steroid biosynthesis. Newer research shows that the final dose required to clinically control Cushing’s syndrome is significantly lower, if a low starting dose is chosen (ECVIM 2012 and 2015). This can be slowly increased if necessary. The manufacturer recommends 2.2-6.7 mg/kg once daily. A single daily dose is sufficient for approximately 80% of all dogs. Dogs with heart failure or diabetes mellitus should, however, always be treated at least twice daily. Therapy control testing using ACTH stimulation tests should be carried out after 10 days, 4 weeks, 12 weeks, and then every 3 months after therapy begin, or whenever the dose is adjusted. The ACTH stimulation test must be carried out 4-6 h after administration of the tablet. Whether a base level cortisol determination 24 h after administration of the medication is more informative is currently under discussion (Ramsey, ECVIM 2015).