Before or at the beginning of the breeding season, special attention is paid to mares that were not in foal or aborted in the past year. In addition to endometrosis, endometritis is the most frequently diagnosed gynaecopathy of the mare in this context. Therefore, a thorough evaluation of its causes is of particular importance.
However, leukocyte infiltration of the endometrium is not a pathological finding per se; after breeding/insemination, for example, there is often a transient inflammatory reaction which subsides within 24 – 48 hours post inseminationem.
Thus, endometritis refers to all inflammatory processes that exceed the physiological selfcleansing process of the endometrium, regardless of their aetiology. In most cases, the general condition of the mares is not disturbed.
The inflammatory reactions can be classified based on different aspects:
1 Clinical appearance
1.1 Acute catarrhal endometritis is accompanied by gradually variable fluid accumulation, which can be clinically detected by rectal palpation of the uterus, sonographic examination or by gross sensory and/or cytological examination of the vaginal discharge.
1.2 Chronic lymphoplasmacellular endometritis (endometritis sicca) eludes clinical diagnosis because there is no exudation of inflammatory cells into the uterine lumen. Furthermore, bacteriological examination often yields negative results, as it is apparently caused by a chronic local immunological dysregulation. Thus, the gold standard for the thorough diagnosis of endometritis sicca is the histological examination of an endometrial biopsy (see below).
2 Aetiology
2.1 Infectious endometritis includes venereal infections, such as equine dourine caused by Trypanosoma equiperdum, which, however, is currently considered to be eradicated in Central Europe. Contagious equine metritis (CEM) is caused by the bacterium Taylorella equigenitalis and is a notifiable disease. Detection is either done by culture or by PCR. In addition, various other bacterial genera may also be involved in the development or maintenance of endometritis.
From 6291 uterine swabs sent in to Laboklin for bacteriological examination in 2019, pathogenic bacteria were detected in approximately 26% of the samples. Most of them (84%) were ß-haemolysing streptococci; Klebsiella sp. (5.5%), E. coli var. haemolytica (5.19%), Staph.aureus (2.63%) or Pseudomonas aeruginosa (2.15%) were less frequently detected.
Particularly Pseudomonas sp. and E. coli have the ability to form biofilms in the uterus, which can result in a decreased response to antibiotic treatment.
Less often, endometritis is caused by fungal infections, but a mycological examination of a uterine swab should be requested, for example, after a long period of antibiotic treatment. Yeasts of the genus Candida play the biggest role here, moulds such as Aspergillus sp. are less frequently detected.
- Picture: A. Reiher
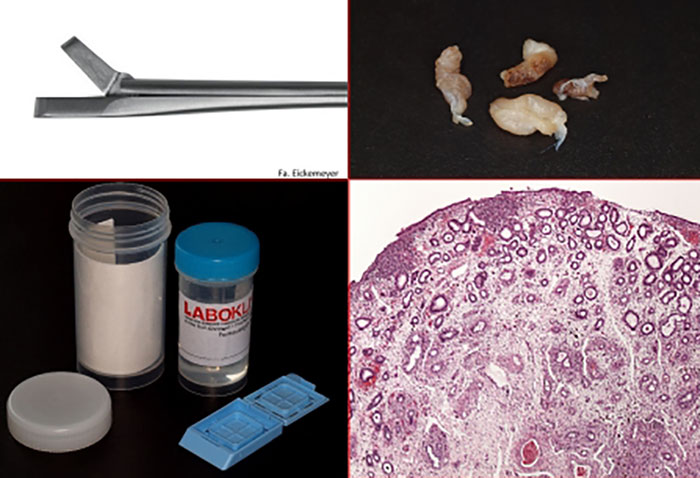
- Fig. 1: Endometrial samples with a diameter of approx. 0.5 cm (top right) are obtained using uterine biopsy forceps (top left) and fixed in 10% buffered formalin (bottom left) immediately after collection. After preparing the samples in the laboratory, it is possible to perform a histological characterisation of the inflammatory processes (bottom right).
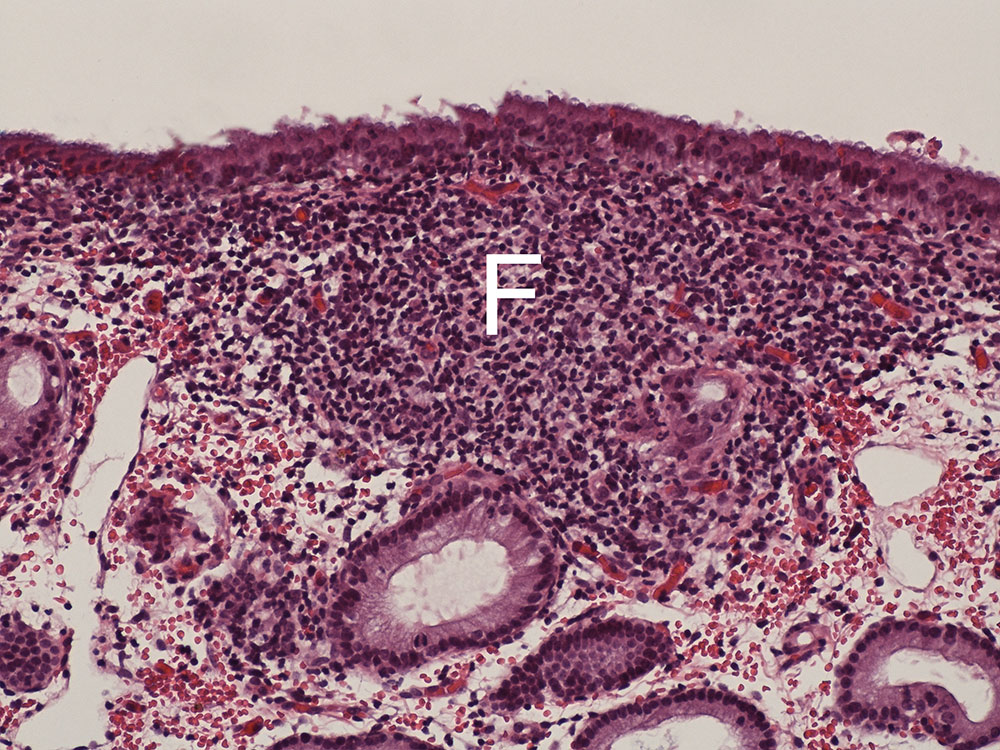
- Fig. 2: Chronic follicular (F) lymphoplasmacellular endometritis without the formation of exudate (HE staining, 200x)
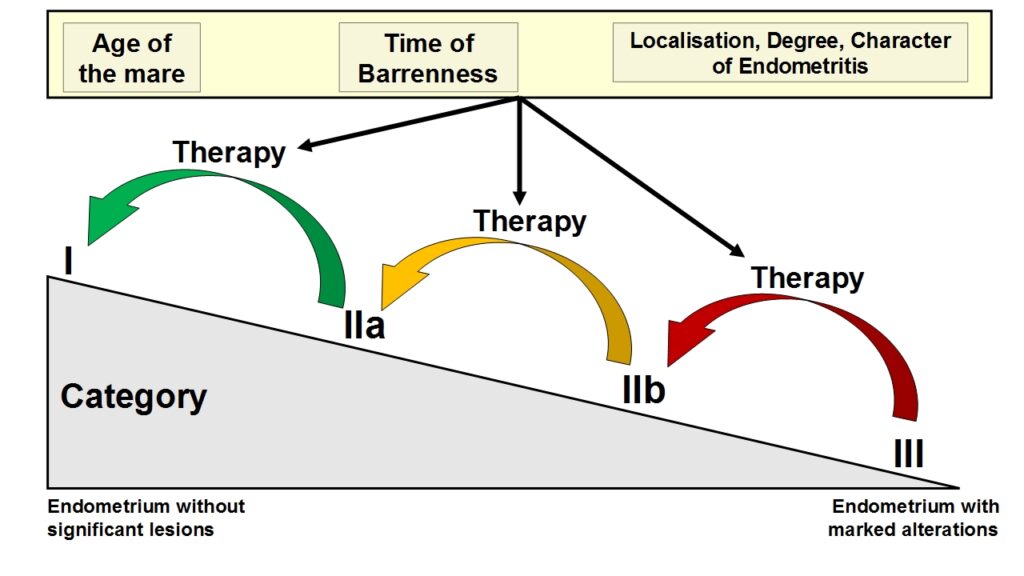
- Fig. 3: Diagram of an improved fertility prognosisafter successful treatment of endometritis takinginto account clinical-gynaecological findings.Histologically, severe inflammation can bedetected in category III, whereas in category I,leukocyte infiltration of the endometrium is lowor no longer clinically relevant.
2.2 Predisposing factors for the occurrence of non-infectious endometritis are anatomical changes of the external genitals, e.g. vulvar cleft, scarring after difficult foaling, a cranially sunken anus as a result of reduced body fat, etc., which facilitate the entry of air or urine into the vagina or even the uterus.
The most common reason for non-infectious endometritis, however, is breeding/insemination which causes an inflammatory reaction of the endometrium (breeding-induced endometritis). Physiologically, it is a transient inflammation which subsides within 48 hours due to effective uterine clearance.
Yet, some mares are more susceptible to developing persistent breeding-induced endometritis (so-called “susceptible mares”), so that after contact of the endometrium with the semen, inflammation occurs with massive accumulation of fluid which cannot be eliminated from the uterus. Thus, persistent endometritis develops, which leads to early embryonic loss.
3 Laboratory diagnostics Bacteriological, cytological and molecular genetic examinations:
If, during the special clinical-gynaecological examination, vaginal discharge or traces of secretion in the area of the external genitals are detected, which indicate an exudative inflammation in the genital tract, it is recommended to take a sterile endometrial swab and to examine cytological samples.
Taking a sterile endometrial swab for bacteriological examination is best done using instruments such as a speculum, cervical forceps and swab collection systems, as this method has the lowest risk of contaminating the swab with bacterial flora of the vagina or the external genitals. Furthermore, this method ensures a high diagnostic reliability of the bacteriological examination. Immediately after collection, the swab should be put into a transport medium (Amies medium) and labelled to avoid any confusion. Samples should be cooled and transported to the laboratory overnight. After collecting the endometrial swab, it is advisable to take a sample of the endometrium for cytological (and possibly histological) examination.
For the detection of Taylorella equigenitalis, two swabs (in Amies medium with charcoal) must be taken from the clitoral fossa and the clitoral sinus (according to Directive 92/65/ EEC), but not from the endometrium.
It is always best to submit the swab immediately after sampling; in general, no more than 24 hours should elapse before starting the bacteriological examination and no more than 48 hours for a PCR test. Please note that when both methods are combined, two separate swabs must be submitted, one for the PCR test and one for the cultural examination.
Cytology provides information about the character of the secretion. The advantage of this method is that it is quick, easy and noninvasive. Using a double-guarded collection system and a cytology brush, secretion and cells are collected from the surface of the endometrium and rolled onto a slide which is air-dried and microscopically evaluated in the laboratory using Diff-Quik staining. If large amounts of mucus are present in the smear, it may be an indication of mucometra. The detection of neutrophil granulocytes and perhaps also of bacteria or fungi indicates purulent inflammation and, thus, an infection. The advantage of combining a bacteriological and a cytological examination is a significantly increased diagnostic sensitivity as well as the possibility to better assess false negative bacteriological results (e.g. due to nonrepresentative sampling).
The examination methods presented so far are suitable for identifying catarrhal-purulent endometritis as the sampling techniques are non-invasive.
By contrast, inflammatory processes which are not associated with exudation of leukocytes into the uterine lumen, can neither be diagnosed clinically nor by bacteriology or cytology.
Endometrial biopsies (histology)
For a comprehensive assessment of chronic and/or deep endometrial inflammatory processes, the histopathological examination of endometrial biopsies is the method of choice. In barren mares, it is therefore recommended to do a biopsy combined with a bacteriological examination at the beginning of the season.
Tissue samples of the endometrium are collected vaginally using biopsy forceps. The endometrial sample is immediately placed in 10% buffered formalin (Fig. 1). Even though this is a (slightly) invasive method, mares usually show no reaction to the sampling procedure, as the endometrium is a tissue with a high regenerative capacity.
During the histopathological examination of the biopsies, the degree, character, site, extent and timing of the inflammatory response are recorded. First of all, this allows for a targeted analysis of the cause (e.g. purulent inflammation – bacteria/fungi; eosinophilic inflammation – semen diluent, flush, foreign bodies; lymphoplasmacellular inflammation (Fig. 2) – chronic local immune response), and secondly, these criteria are relevant for an epicritic evaluation of the lesions in terms of treatment success and, ultimately, the prognosis of fertility.
Fertility prognosis according to Kenney & Doig (1986) and according to Schoon et al. (1992 and 1997):
Inflammatory lesions of the endometrium are included in the categorisation according to Kenney & Doig (1986), but the clinical picture of the inflammatory alteration, its histopathological character as well as its reversibility are not taken into account. However, since these factors are essential for a comprehensive interpretation, they will be discussed in more detail in the epicritic evaluation of the results (Schoon et al. 1992 and 1997) (see Fig. 3).
The categories according to Kenney & Doig (1986) are correlated with the statistical probability that the mare conceives and successfully delivers the foal (see Tab. 1).
| Category | Foaling probability |
| I | 80 – 90% |
| IIa | 50 – 80% |
| IIb | 10 – 50% |
| III | < 10% |
Tab.1: Categorisation based on histopathological findings on the endometrium according to Kenney and Doig (1986)
The categorisation represents a current assessment of the expected breeding success at a given time; however, it is important to note that – depending on the type of endometritis and treatment success – the category can be improved (see Fig. 3).
Therefore, the age of the mare, the time of barrenness, the site of the endometritis and its character are also taken into account in the epicritic evaluation of the histopathological findings. The aim is to provide practicing colleagues as much diagnostic information as possible in order to decide whether or not targeted treatment has a chance of success.
Dr. Kathrin Jäger