Sodium (Na)
Sodium is the most important cation in the extracellular fluid. Hence, it is essential for maintaining osmolality or the distribution of water between the extracellular space (ECS) and the intracellular space (ICS). Hypo- and hypernatraemia occur when the sodium/water ratio in the ECS shifts towards water or sodium. This is often caused by an increase or decrease in total body water without an absolute change in electrolytes. But since Na is the main component of osmotically active substances in the ECS, hypo- and hypernatraemia are associated with changes in osmolality.
Na is the major electrolyte in the ECS and potassium (K) in the ICS. This asymmetric distribution of the electrolytes across the cell membrane requires active exchange of both cations by the Na/K-ATPase.
As a result, body fluids are in osmotic equilibrium. The distribution of water between the ECS and the ICS is normally constant and only shows slight fluctuations of just 1 – 2%. Acute changes in serum Na concentration which are not accompanied by a corresponding change in intracellular K concentration cause permeation of water from the ECS to the ICS. Cellular oedema occurs.
Concentrations of Na in serum and in the interstitial fluid are almost identical. Na causes about 95% of the osmotic pressure. The organism regulates the plasma Na concentration by adjusting the water content of the ECS and keeping the total body Na and Na in serum constant within a narrow range. This is done by drinking or by renal excretion of free water.
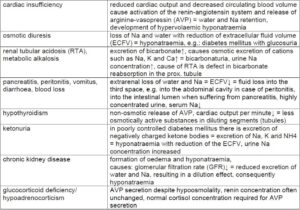
Examples of diseases and causes which can lead to hyponatraemia
(Tab. 1)
Examples of diseases and causes which can lead to hypernatraemia
(Tab. 2)
Potassium (K)
In terms of quantity, potassium is the most important intracellular cation. More than 98% of the potassium in the body is found inside the cells. The serum potassium concentration is regulated within narrow limits. It is very important for membrane potentials; disorders of potassium balance lead to dysfunctions of skeletal muscles, heart and nerve cells. Potassium homeostasis is regulated by oral intake, distribution between the ECS and the ICS and renal elimination.
The regulation by the Na/K-ATPase is an important control mechanism for the movement of potassium between the ECS and the ICS. About 90% of the potassium is excreted via the kidneys, only a small part via the intestines.
Even though the plasma K concentration is only a moderate indicator of total body potassium, it is physiologically important for assessing the transmembrane electrochemical gradient.
- Tab. 1: Examples of diseases and causes which can lead to hyponatraemia
- Tab. 2: Examples of diseases and causes which can lead to hypernatraemia
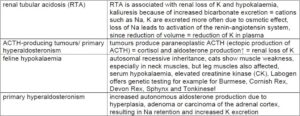
- Tab. 3: Examples of diseases and conditions which can lead to hypokalaemia
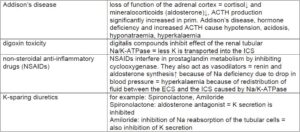
- Tab. 4: Examples of diseases and conditions which can lead to hyperkalaemia
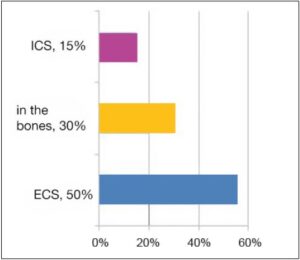
- Fig. 1: Chloride fractions
- Tab. 5: Example of diseases associated with an increase in serum chloride
- Tab. 6: Examples of diseases associated with a decrease in serum chloride
Examples of diseases and conditions which can lead to hypokalaemia
(Tab. 3)
Examples of diseases and conditions which can lead to hyperkalaemia
(Tab. 4)
Chloride (Cl)
Chloride is one of the most important anions in the ECS. To a large extent, it is bound to sodium and present as common salt (NaCl). As a counterion of Na, it plays an important role in maintaining the water distribution between the ECS and the ICS, hence, in the plasma osmolality.
As a vital electrolyte, more than half of the chloride is found in the ECS (about 55%), approximately one third in the bones (about 30%) and only a small part inside the cells (about 15%).
Chloride fractions
Chloride is mainly taken in through common salt (sodium chloride) in food. It is excreted via the kidneys and regulated by the hormone aldosterone, which causes reabsorption of the anion if there is a lack of chloride (Fig. 1).
Example of diseases associated with an increase in serum chloride
(Tab. 5)
Examples of diseases associated with a decrease in serum chloride
(Tab. 6)
Dr. Anja Cölfen