The body of mammals is colonised with countless microorganisms such as bacteria, fungi, protozoa and viruses. Colonised areas include the oral and nasal cavities, the surface of the skin as well as the mucous membranes of the urogenital and gastrointestinal tracts. The individuals that form this collective are called the microbiota, the accumulated genetic information of each individual microbe is called the microbiome. With 1011 – 1012 bacteria per g of faeces, the primary colonisation site of the microbiota is the colon. Of these bacteria, more than 99% are strictly anaerobic and, thus, make up the main part of the intestinal flora.
If someone wanted to examine the composition of this community more closely, there were almost only microbiological-cultural methods available until the end of the nineties. Culturable intestinal bacteria could be detected this way, however, with less than 1%, they just represent a very small proportion of all microbes in the intestine. Thus, the much larger proportion of non-culturable microbes remained largely unnoticed. Only the development of new test methods on a molecular biological basis (e.g. next-generation sequencing) made it possible to also detect bacteria that could not be microbiologically cultivated. Thanks to this approach, the intestinal microbiome could be examined in all its complexity for the first time, which led to a real microbiome boom in science. By now, numerous studies have proven that intestinal microbes definitely make an important contribution to the health of the human host.
The most important tasks of the intestinal flora include: utilisation and digestion of food, synthesis of essential micronutrients (e.g. vitamin B12), maintenance of the intestinal mucosal barrier, regulation of the intestine-associated immune system, defence against pathogens and opportunistic pathogens and promotion of healthy digestion and intestinal motility.
Intestinal microbiome of dogs and cats
Compared to the human intestinal microbiome, scientist rather seldom focus on the intestinal microbiome of small animals such as dogs and cats. The pathomechanisms initiated and/or promoted by dysbioses of the intestinal flora are very similar. More and more often, diseases like chronic diarrhoea, IBD, food allergy, metabolic disorders and atopic diseases are seen in the veterinary practice (Fig. 1).
In fact, studies by an American research group show that the differences between human and canine microbiome are much smaller than previously assumed. The genetic information of all intestinal bacteria is 63% identical in people and dogs. (In mice or pigs, by contrast, the similarity is much lower, at 20% and 33%). One reason often mentioned for this is the close social bond pet owners build with their pets. In addition to spatial proximity, there is often similar nutrition (e.g. feeding from the table), which significantly increases the probability of a horizontal transfer of bacteria in both directions. Further proof of this hypothesis is that the overlap of intestinal microbiomes from dog owners to their own pets is significantly higher than to foreign dogs that do not live in the same household.
It is still quite unclear whether such similarities of the intestinal microbiome also exist in dogs and cats. Is the intestinal flora of a dog with dysbiosis comparable to that of a cat? And if this is not the case: How does a dysbiotic condition of a dog differ from that of a cat?
-
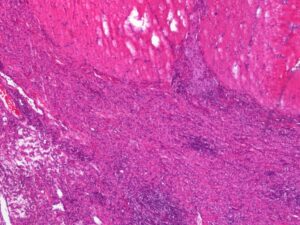
Fig. 1: High-grade chronic histiocytic to mixed-cell colitis in a Boxer (haematoxylin-eosin stain, 4x).
Source: Dr. Ulrike Schwittlick, Laboklin GmbH & Co. KG
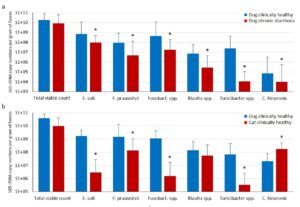
- Fig. 2: (a) Microflora composition in dogs with chronic diarrhoea compared with the clinically healthy control group (n = 30, * p<0.05, Wilcoxon-Mann-Whitney test). (b) Microflora composition in clinically healthy dogs in comparison with clinically healthy cats (n = 27, * p<0.05, Wilcoxon-MannWhitney test).
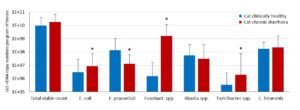
- Fig. 3: Microflora composition in cats with chronic diarrhoea compared with the clinically healthy control group (n = 27, * p<0.05, Wilcoxon-MannWhitney test).
- Fig. 4: Copy numbers of lactobacilli, bifidobacteria and enterococci in clinically healthy dogs and cats as well as in dogs and cats with chronic diarrhoea (n = 25, no significant differences).
Intestinal dysbiosis in dogs
Our own studies, which compared the copy numbers of important anaerobic bacterial markers for intestinal dysbiosis in dogs with chronic diarrhoea and a clinically healthy control group by means of quantitative real-time PCR, show clear differences (Fig. 2a). Particularly noticeable is a significant reduction in the anaerobic, carbohydrate-digesting intestinal flora, the saccharolytic bacteria (Faecalibacterium prausnitzii, Blautia spp., Turicibacter spp.). An important function of these groups of bacteria is to metabolise fibre components in the food that are difficult to digest to short-chain fatty acids such as acetate, propionate and butyrate. They serve as the main source of energy for the enterocytes in the colon and are essential for the maintenance of the muscosal barrier. Regarding their diet, dogs are omnivores. Their digestive system and metabolism are therefore adapted to a high carbohydrate content in their food. This is why a shift in the intestinal flora towards proteolytic bacteria (e.g. Clostridia, Proteus, Klebsiella) and with reduced numbers of saccharolytic bacteria is not a physiological state but a sign of intestinal dysbiosis.
… and in cats
Although the anatomical similarities of the gastrointestinal tract suggest otherwise, the composition of the intestinal microbiota is very different in dogs and cats. Figure 2b shows a comparison of the intestinal microbiome of clinically healthy dogs and cats. There is a significant underrepresentation of saccharolytic intestinal microorganisms in cats (E. coli, Fusobacterium spp., Turicibacter spp.). As in dogs, the reason for this is probably the diet. Domesticated cats are obligatory carnivores and need protein-rich animal tissue to meet their energy needs.
Therefore, their digestive system and metabolism are adapted to a higher protein and lower carbohydrate content in the food. The resulting nutrient supply in the intestine is an enormous selective advantage for proteolytic bacteria and a disadvantage for saccharolytic ones. A eubiotic status of the microbiome in a healthy feline gut is thus characterised by a lower number of saccharolytic and a higher amount of proteolytic bacteria.
Disorders of the intestinal flora in cats
Just as in dogs, there are also measurable differences in the composition of the intestinal microbiome between clinically healthy cats and animals with chronic diarrhoea. A comparison of the microbial dysbiosis markers of the two groups shows that the number of saccharolytic intestinal bacteria is significantly increased in cats with chronic diarrhoea compared to clinically healthy animals. This could mean that cats depend less than dogs on microbial fermentation products of saccharolytic bacteria. A shift in the bacterial balance towards saccharolytic intestinal bacteria is therefore more characteristic of a dysbiotic situation in the intestine.
Similarities and differences
Despite the differences between dogs and cats regarding their diet and intestinal microbiota, there seem to be certain intestinal bacteria in both species that are essential for maintaining intestinal homeostasis. An example of this is Faecalibacterium prausnitzii. This is a strictly anaerobic gram-positive bacterium with important anti-inflammatory and protective effects on the intestinal mucosa. Reduced copy numbers of this intestinal bacterium are a clear indication of intestinal dysbiosis in dogs and cats.
The opposite seems to be true for bacteria of the species Fusobacterium. In dogs, viable counts of Fusobacterium spp. are significantly higher in the clinically healthy control group than in animals with chronic diarrhoea; in cats, in contrast, this is only true in the affected group (Fig. 2b). It is not clear whether these are different bacterial counts of the same species or of different bacterial species. The environmental conditions in the dysbiotic cat gut may possibly promote the growth of fusobacteria with pro-inflammatory potential, which could favour the occurrence of clinical symptoms.
Indications to test for dysbiosis
Even though there are significant differences in the gastrointestinal tract between dogs and cats, the indications for a micro-ecological analysis of the intestinal microbiome are identical in both species. They include:
- gastrointestinal disorders of unknown origin, e.g. chronic diarrhoea and flatulence
- digestive disorders, exocrine pancreatic insufficiency
- recurrent parasitoses (e.g. Giardia)
- food intolerances and allergies
- status monitoring after antibiotic treatment
A micro-ecological analysis of the intestinal flora can support the differential diagnosis and provide valuable new therapeutic approaches. However, only microbiological-cultural methods are often used for such tests, for example when determining the bacterial counts of enterococci, bifidobacteria or lactobacilli. For several reasons, these tests are not sufficiently suitable for a diagnosis. On the one hand, with less than 1%, only a very small proportion of the intestinal bacteria is detected. And on the other hand, when comparing the copy numbers of these bacteria in both dogs and cats, there are no significant differences (Fig. 4). This is also true when comparing clinically healthy animals and animals with chronic diarrhoea.
The microbiologically-based, culture- independent analysis of dysbiosis, in contrast, tests and quantifies essential, non-culturable bacterial markers for intestinal dysbacteria. If reference values are adapted, which consider the differences between the normal flora of dogs and cats, intestinal dysbioses can be detected quickly and reliably.
Treatment of intestinal dysbiosis
Changing to low-fat, highly digestible food while taking into account the individual nutritional requirements is the most important step in the treatment of disorders of the intestinal flora. The risk of inflammatory responses and intolerance reactions can be reduced by avoiding raw food and allergen-rich food ingredients. In case of acute diarrhoea, faeces-forming, toxin-binding substances such as aluminosilicate clays or humic acids can effectively relieve the symptoms.
Ground psyllium husks added to the food have a prebiotic effect and promote the growth of the protective flora in a natural way. Additionally, in combination with water, they release mucilage that slows down the intestinal transit in case of diarrhoea and facilitates defaecation in case of constipation.
Only when the acute symptoms have subsided, the use of microbiological therapeutics is indicated. Attention should be paid to always use probiotics in a suitable dosage form (enteric-coated capsules or tablets, microencapsulated powder), high bacterial counts (> 109 CFU per dose) and with a high diversity of bacterial species involved.
For all gastrointestinal disorders associated with chronic diarrhoea, oral immunotherapy with autovaccines is also a suitable therapeutic option. Through the increased production of specific antibodies and a supplementary supply of mucosa-nutritive metabolites, the mucosal barrier is supported and the immune defence is strengthened. Its use is recommended both in acute condition and as a cure to prevent recurrence.
Literatur
-
MK AlShawaqfeh, B Wajid et al.: A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiology Ecology 2017;11:136
-
L Coelho, J Kultima et al.: Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 2018; volume 6, 72