As the largest organ of the body, the skin is easily accessible for various examinations, yet the diagnostic work-up of dermatology patients can be extremely frustrating. It is therefore best to always examine dermatology patients according to a fixed diagnostic plan. A full medical history – if possible by means of a case history form – and a clinical dermatological examination are followed by a more detailed assessment of the lesions detected in the dermatological examination. A microscopic examination of material from the skin surface or from hair is the first step in further diagnosis. This way, bacteria, fungi, yeasts or parasites as well as inflammatory cells can quickly be identified.
Generally, sampling can be carried out easily, but the efficiency of the possible tests greatly depends on selecting the right sample material as well as on how the samples are taken.
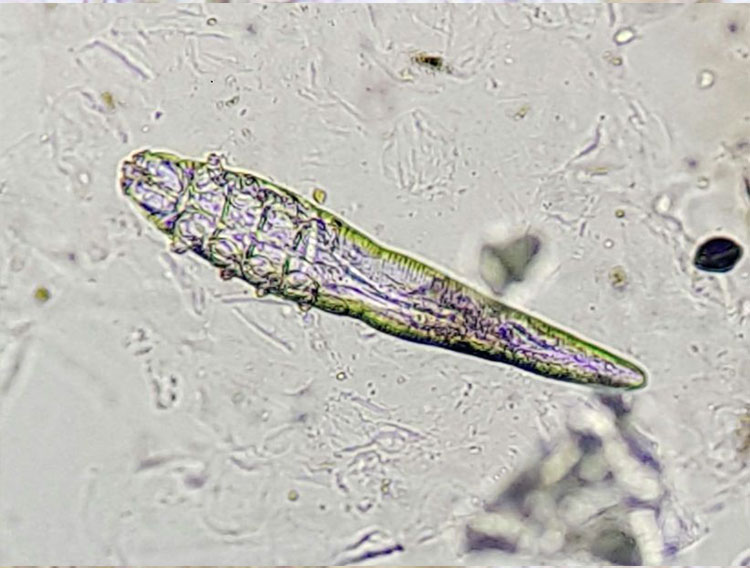
A tape test is the best method for detecting Cheyletiella (Fig. 1). These mites cause dry seborrhoea, especially on the back. The sample material is obtained by repeatedly pressing the adhesive tape on the affected skin areas. Alternatively, scales can first be collected with a comb or, if there is excessive scaling, the material can also be collected directly from the examination table. Then, a drop of paraffin oil is applied to a microscope slide and the adhesive tape is stuck over it. The tape preparation is completely sampled with the 4x objective while the condenser is swung out/lowered, as there are usually only a few mites present.
To detect Sarcoptes mites and the less common Notoedres mites in cats, a superficial skin scraping is done. Cheyletiella, which also live on the skin surface, can sometimes be detected in the superficial skin scraping; less frequently, Demodex mites or fungal infection of hair can be found this way.
Take a used/blunt scalpel blade (to minimise the risk of injury) and paraffin oil to scrape off as many scales as possible from the previously shaved skin areas. Since Sarcoptes mites are usually only present in small numbers, it is recommended to collect the sample material from several sites (especially from the edges of the ears and on the elbow) and a large area. Moreover, sampling fresh papules increases the diagnostic accuracy.
The sample obtained is placed on a slide with another drop of paraffin oil, covered with a coverslip and examined under a microscope (4x or 10x objective with the condenser swung out or lowered).
For the diagnosis of sarcoptic mange, PCR on skin scrapings (without paraffin oil) or the determination of serum antibody titres are also available.
Deep skin scrapings are used to diagnose demodicosis.
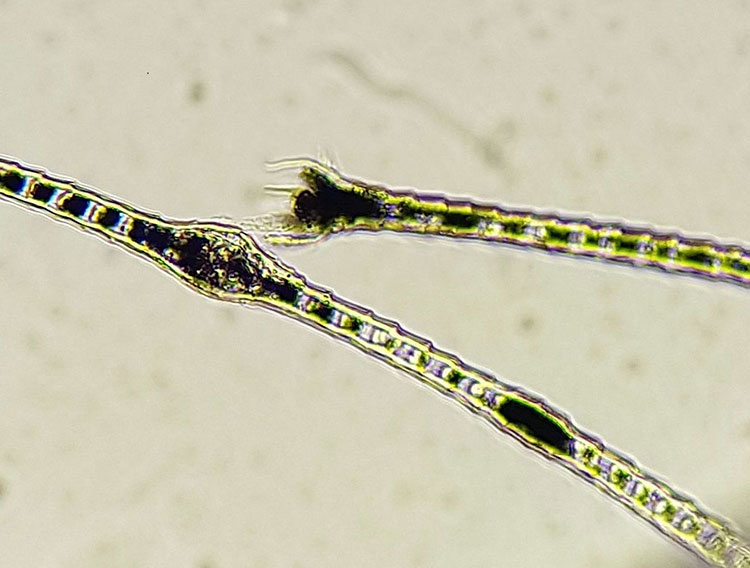
With a scalpel blade or even a sharp spoon and paraffin oil for moistening, the skin is scraped in the direction of hair growth until capillary bleeding occurs. Between scrapings, the skin should be squeezed repeatedly to bring the mites from the depth of the hair follicles to the surface. It is best to sample those areas that exhibit efflorescences such as redness, alopecia, scaling, comedones or follicular casts. The scraped material is immediately spread on a slide and mixed well with the oil as the sample material could coagulate into lumps due to the bleeding and thus become more difficult to analyse. A coverslip is placed on top and the sample is then examined with a 4x or 10x objective. Demodex mites (Fig. 2) are sometimes present in large numbers and can also often be found along the hair roots on the specimen.
Apart from microscopic detection, there is also a PCR test available for the diagnosis of demodicosis which covers all species described in dogs (D. canis, D. injai, D. cornei) and cats (D. cati, D. gatoi, D. felis).
In some cases, skin scrapings are also recommended for cytological examinations if the skin surface is intact. However, these specimens are often not diagnostic as the cells are usually completely destroyed during sampling.
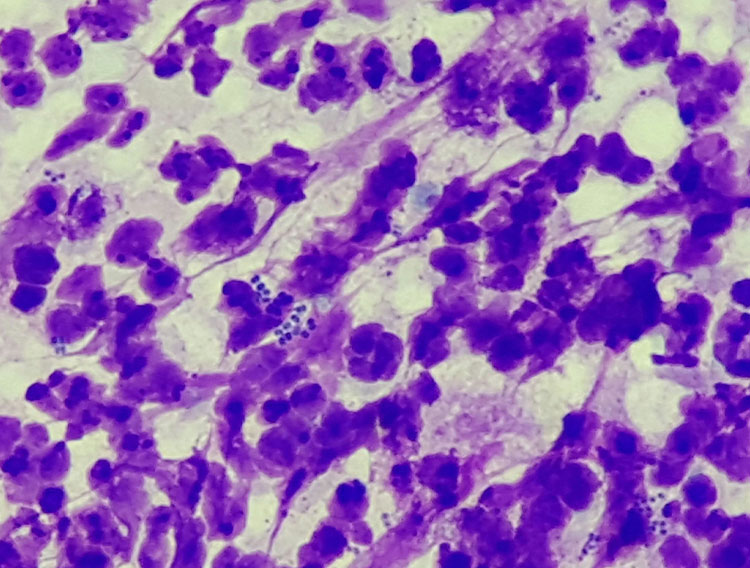
Impression smears for the cytological examination of the skin surface are prepared particularly with regard to the type of inflammation involved or secondary infections present (Fig. 3). A slide is pressed onto the skin lesion and then air-dried. When making an impression smear, it is necessary to ensure that the slide is not wiped over the skin lesions, as this destroys the cells and only nuclear remnants and chromatin fibres will be visible under the microscope. As in surgery, the motto “dab, don’t wipe” also applies here. In the microscope, the specimen is first scanned at low magnification (10x or 20x objective) to find diagnostically conclusive areas which are then evaluated in detail at high magnification (40x to 100x, oil immersion).
- Fig. 1: Fur mite (Cheyletiella spp.); Cheyletiella live on the skin surface and feed on tissue fluids. The entire life cycle takes place on the host, so the eggs of the mites can also be found on the hair.
- Fig. 2: Dog follicle mite (Demodex canis); Demodex mites live in the hair follicles and feed on sebum as well as shed cellular material. They can be found in small numbers in many healthy animals (and also in humans).
- Fig. 3: Pyoderma; in addition to numerous degenerated neutrophils, intracellular cocci are also seen
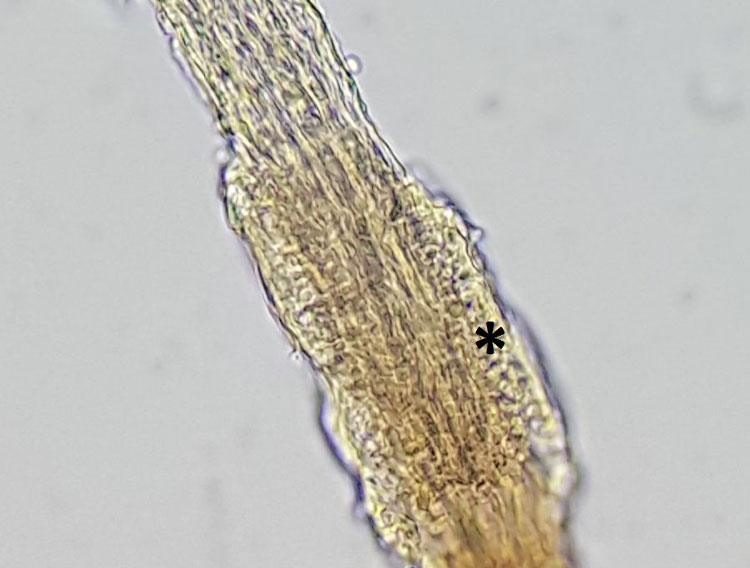
- Fig. 4: Dermathophytosis; the hair shaft is streaked with fungal hyphae; round fungal spores are visible at the edge (asterisk).
- Fig. 5: Colour dilution alopecia (CDA); macromelanosomes are located in the hair shafts and can cause the hair to break off
However, if these sites are difficult to access (e.g. in between the toes) or if they are too dry for any material to adhere to the slide, the tape test method can be used. The transparent adhesive tape is pressed repeatedly onto the affected skin area until sufficient material adheres and the tape is no longer sticky. Fixation is not required for staining as the sample is already fixed by the adhesive tape and, in addition, adhesive tapes often get cloudy when alcoholic fixative solutions are used. A drop of oil is placed between the stained adhesive tape and the slide and a microscopic examination is carried out for microorganisms (bacteria/rods/cocci or yeasts) and inflammatory cells.
Very rarely, indications of other skin diseases (e.g. cutaneous lymphoma, pemphigus) may be found with the tape test method. However, as it is much more difficult to assess the cells on the adhesive tape than on a slide, tape impressions should rather be used when testing for secondary infections and inflammatory reactions.
For trichoscopy, hairs are plucked out with a clamp or tweezers, placed on a microscope slide with a little oil and examined under the microscope after covering them with a coverslip. In every patient with suspected fungal skin disease, the hairs should also be examined microscopically at the same time as the sample is taken for culture or PCR (Fig. 4).
In some cases, dermatophytosis can promptly be diagnosed and treatment initiated immediately. Yet, culture examination or PCR always needs to be carried out: on the one hand because the sensitivity of microscopic detection is not very high and on the other hand because the type of dermatophyte cannot be determined by trichoscopy.
For the examination of asymptomatic animals or for therapy monitoring after healing of the efflorescences, the material for fungal culture is best obtained by the McKenzie brush technique.
With a new toothbrush, the coat is brushed for at least five minutes, during therapy monitoring particularly in those areas that were originally affected.
Demodex mites can also be detected by trichoscopy if the preparation of a deep skin scraping is not possible due to the site or the patient’s lack of compliance. The sensitivity of deep skin scrapings is significantly higher, though. Since the Demodex mites live directly in the hair follicle at the hair roots, they can often be plucked out with the hair. It is important, however, not to squeeze the skin unlike it is done in a scraping, because this way the mites are pushed out of the hair follicle and no longer sit at the root of the plucked hair.
A trichogram in the narrower sense (detailed trichoscopic examination of the morphology of hair) can be helpful especially in the diagnosis of non-inflammatory alopecia. Using a clamp or tweezers, with their tips being covered with rubber tubes to avoid iatrogenic damage to the hairs, hairs are plucked from altered sites. Strictly speaking, 100 hairs should be embedded in paraffin oil in parallel on a microscope slide and covered with a coverslip. The roots, hair shafts and the tips of the hairs are assessed in a microscope at low magnification (4x or 10x objective). If there are many hair roots in the telogen stage, it indicates the presence of an endocrine disease (hypothyroidism, hyperadrenocorticism). Malformed hair roots lead to the suspected diagnosis of follicular dysplasia.
Patients with colour dilution alopecia and black hair follicular dysplasia show melanin clumps in the hair shafts (Fig. 5), which will eventually lead to the affected hair breaking off. The appearance of the hair tips provides important information as to whether alopecia may have resulted from automutilation. Particularly cats often do not show their pruritus-induced over-grooming in front of their owner. If broken hair tips and split ends are found in the trichogram, it can be assumed that a pruritic disease is present after all.
Conclusion
Skin diseases always require a systematic diagnostic work-up. With the help of simple and inexpensive basic examinations, a diagnosis can sometimes be made very quickly or at least it is possible to obtain indications for the further course of action. Diagnostic accuracy is especially high if the correct way of sampling is used for the particular examination.
Dr. Maria Christian
| Suspected diagnosis | Sampling | Note | Further examinations |
| Sarcoptes | superficial skin scraping | fresh papules, scales edge of the ear + elbow | PCR on scraping and determination of serum antibody titre |
| Cheyletiella | tape impression possibly superficial skin scraping | especially on the back | |
| Demodex | deep skin scraping, possibly plucked hairs/trichoscopy | areas that are red/hairless or show comedones or follicular casts | PCR on scraping/hairs |
| Dermatophytes | plucked hairs/trichoscopy possibly superficial skin scraping,
McKenzie brush technique |
McKenzie brush technique in asymptomatic animals | always with culture examination or PCR |
| Secondary infections | microscope slide impression smear, tape impression if the skin surface is dry/normal or if sites are difficult to access | scrapings are not suitable because of cell destruction | bacteriological or mycological examination |
| Non-inflammatory alopecia | trichogram (approx. 100 hairs with root and tip) | cover clamps/tweezers with rubber tubes | hormone profile, histopathology (biopsy) |
| Automutilation | trichogram (approx. 100 hairs with root and tip) | cover clamps/tweezers with rubber tubes | clarification of pruritus
|