These are multiple causes of alopecia in small mammals. Endocrine causes are considered after ruling out ectoparasitic diseases. It requires a detailed history of the process (duration, course, existing pruritus, vaccination status, husbandry [indoors, outdoors], protection against insects, lesions on contact animals or owners), and a clinical and dermatological examination (combing, swab/adhesive strip method, skin scrapings, bacteriological and mycological examination, PCR, antibody determination, etc.).
Diseases of the thyroid gland
When a small mammal presents with alopecia without pruritus, thyroid problems are usually thought to be present, although these are rarely causative. Guinea pigs with hyperthyroidism only show secondary alopecia (mite or paraneoplastic), which appears late in the disease course and usually with other previous clinical signs.
Hyperthyroidism in guinea pigs is similar to feline hyperthyroidism and occurs occasionally. The increased thyroxine (T4) secretion is caused by thyroid hyperplasia or neoplasia (adenoma, adenocarcinoma). The median age of presentation is five years. Typical clinical signs are weight loss despite appropriate food intake (95%), increased ventral neck circumference (45%) and behavioural changes (18%) such as hyperactivity, restlessness, jumpiness, prolonged sleep and isolation from conspecifics. Later in the course of the disease, polyuria (21%), alopecia secondary to mites (18%), loss of appetite (16%), loose stools (13%) and tachycardia (8%) develop. The clinical diagnosis of hyperthyroidism is made by determining an elevated T4 concentration. There is no correlation between increased T4 concentration and palpable circumferential neck enlargement. There is also no correlation between T4 concentration and sex. However, older guinea pigs show significantly lower T4 concentrations than young and middle-aged animals, probably due to the more frequent underlying diseases in older animals. For therapy control, it is recommended to determine the T4 concentration again after 3 to 4 weeks of treatment. Since sick guinea pigs respond differently to the treatment, the medication should be adjusted according to the clinical presentation and not only according to the T4 concentration.
There are no descriptions of primary hypothyroidism in small mammals in the literature. However, there are some studies on non-thyroidal illness syndrome or euthyroid sick syndrome, a downregulation of thyroid hormones due to other diseases (obstructive ileus, dental disease, limb fracture, urinary tract disease, etc.).
Low T4 concentrations should, therefore, never be assessed without clinical and further investigations (blood count, clinical-chemical parameters).
Diseases of the adrenal glands
Adrenal gland diseases with dermatological manifestations in small mammals are Cushing‘s syndrome (hypercortisolism) in guinea pigs and hamsters and hyperadrenocorticism (HAC) in ferrets. In Cushing‘s syndrome, an increased amount of cortisol is produced in the zona fasciculata. In contrast, in ferret hyperadrenocorticism (HAC), there is increased synthesis of sex hormones and not cortisol in the zona reticularis of the adrenal cortex. Therefore, the HAC of ferrets should not be called Cushing‘s syndrome!
-
Dwarf rabbit and Guinea Pigs
Photo credits: envatoelements
-
Fig. 1: Salivette®, Sarstedt
Photo credits: Laboklin
-
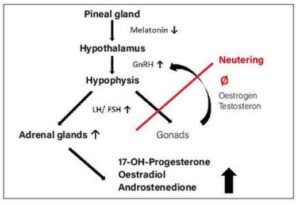
Fig. 2: Pathogenesis of hyperadrenocorticism in ferrets
Photo credits: Jana Liebscher
-
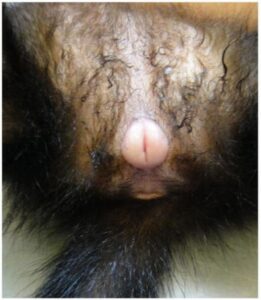
Fig. 3: Vulvar swelling of a female ferret with hyperestrogenism
Photo credits: Dr Jutta Hein
Cushing‘s syndrome (hypercortisolism) is rare in guinea pigs. Due to a higher frequency of pulsatile ACTH release, the basal blood cortisol concentration in guinea pigs is several times higher than in dogs and cats. Hence the term hypercortisolism is used in English literature. In addition to adrenal Cushing‘s disease, the pituitary form has also been described. Typical clinical signs are alopecia without pruritus, polydipsia/polyuria, polyphagia, truncal obesity and, as the disease progresses, bilateral exophthalmos, skin atrophy and hyperpigmentation, weight loss and muscle atrophy. Due to the low prevalence, other possible causes should be excluded first. Urinalysis (specific gravity, biochemistry and sediment if necessary), haematology and blood biochemistry are prerequisites. High basal cortisol concentrations are physiological in guinea pigs and may increase due to stress, handling, illness, pregnancy and/or vitamin C deficiency (- 1500 ng/ml). In animals with Cushing‘s disease, cortisol concentrations can reach up to 3500 ng/ml causing determination cortisol in the blood only possible with extensive dilution. Determination of cortisol concentration in saliva may be a good alternative in these cases, as the cortisol concentration in saliva is usually much lower than in blood, and stress-related concentration fluctuations do not occur as quickly. Saliva sampling is described as non-invasive and stress-free, which may not be entirely accurate. Although compared to blood sampling by an inexperienced veterinarian, saliva sampling is less stressful. Contamination of saliva with blood leads to falsely high cortisol concentrations. Salivettes® (Fig. 1) should be used because of their larger sample volume and higher sensitivity. A dexamethasone suppression test (basal sample, subcutaneous low dose 0.01 mg/kg or high dose 0.1 mg/kg dexamethasone, sampling at 4 and 8 hours) is well suited for differentiating between adrenal and pituitary causes. However, no reference values are available for blood or saliva, except in guinea pig saliva, so assessment is based on the dog‘s values. The ACTH stimulation test (baseline sample, 20 I.U. ACTH/animal i.m. and sampling at 4 hours) is, as in dogs, mainly suitable for treatment monitoring.
There are few reported cases of Cushing‘s syndrome in hamsters, with non-pruritic alopecia and polydipsia/polyuria (three teddy hamsters and one golden hamster). Laboratory diagnostic tests have not been established for this species. With a low urine specific gravity, an ultrasound examination of the adrenal glands may be helpful for diagnosis. However, a definitive diagnosis can so far only be made by histopathological study of the affected adrenal glands.
Hyperadrenocorticism (HAC) is relatively common in ferrets. The prevalence varies according to the region and population studied. A US study in 94 ferrets attending a specialised veterinary practice showed a prevalence of 20-25%. In another study in the Netherlands with 1,274 animals, the prevalence was only 0.55%. The onset of clinical signs is on average 3.5 years after castration. There is no sexual predisposition according to the literature. Adrenal lesions are nodular hyperplasia (56%), adenocarcinoma (26%) or adenoma (16%), which are mostly (84%) unilateral. Besides melatonin deficiency in indoor housing (altered day-night rhythm, longer daylight duration) with loss of inhibitory effect on the hypothalamus (GnRH), neutering is considered the main cause of HAC. The lack of negative feedback of gonadal steroids after neutering leads to a permanently high gonadotropin (GnRH) concentration. This stimulates the pituitary gland to secrete LH/FSH, which in turn stimulates the zona reticularis of the adrenal cortex to produce steroid hormones, especially 17-OH-progesterone, oestradiol and/ or androstenedione (Fig. 2). Typical clinical signs are symmetrical alopecia beginning at the base of the tail and extending over the back, with pruritus in up to 40% of affected animals, and behavioural changes such as the reappearance of sex-specific behaviour and aggressiveness. In females, oestrogen production causes enlargement of the vulva, whereas males are more likely to present with dysuria due to androgenrelated prostatic changes (periprostatic and periurethral cysts). Diagnosis is made by clinical examination, ultrasound and blood tests (Adrenal profile ferrets). With the analysis of 17-OH-progesterone, oestradiol and androstenedione in this profile, the diagnostic sensitivity increases to 96%. An increase in the concentration of at least one of these hormones above the reference range is indicative of HAC.
Diseases of sexual glands
Alopecia may also be caused by diseases of the sexual glands, such as ovarian cysts in guinea pigs or neoplasms causing paraneoplastic syndrome. Hyperestrogenism occurs in intact female ferrets, in animals with ovarian remnant syndrome (ORS) and in spayed female ferrets with HAC, resulting in increased oestrogen levels. Since female ferrets have seasonal polyoestrous and induced ovulation, a prolonged oestrus (standing oestrus) and corresponding hyperestrogenism can occur in the absence of mating and a daylight length of more than twelve hours. This results in bone marrow suppression with pancytopenia that can lead to life-threatening anaemia with thrombocytopenia. In neutered females with ORS and in animals of both sexes with HAC, the bone marrow suppression is rather mild. Typical clinical signs are vulval swelling (Fig. 3) and, in animals with HAC and ORS, symmetrical bilateral alopecia beginning at the base of the tail and spreading cranially. Rarely, galactorrhoea and gynaecomastia are observed. Pale mucous membranes are a sign of anaemia, petechiae of thrombocytopenia due to bone marrow suppression. Melena, haematuria and other haemorrhages may also occur. The diagnosis can often be made on the basis of clinical signs together with nonregenerative anaemia and later pancytopenia (thrombocytopenia, leukopenia) and the determination of oestradiol concentration. An ultrasound examination is useful for differentiation from HAC or ORS. In the case of permanent rancidity or ORS, diagnostic therapy with HCG (2 x at intervals of 14 days) leads to swelling of the vulva. Diagnosis can often be made on the basis of clinical signs, together with non-regenerative anaemia and subsequent pancytopenia (thrombocytopenia, leukopenia) and determination of oestradiol concentration. An ultrasound examination is useful to differentiate HAC or ORS. In case of permanent or ORS, diagnostic therapy with HCG (twice at 14-day intervals) causes swelling of the vulva. Ovarian cysts are prevalent in guinea pigs (up to 90%) and can be unilateral or bilateral. The number and size of ovarian cysts increase with age. Females without a male seem to be affected more often.
There are a distinction between rete ovarii, follicular and parovarian cysts, with rete ovarii cysts predominating. The cysts can be hormone-producing. Increased oestrogen production leads to bilateral flank alopecia, and some females in permanent heat are more aggressive. Due to fluctuating oestradiol concentrations in non-spayed animals and the lack of reference values, oestradiol concentration does not help detect hyperestrogenism. Therefore, the diagnosis is made by clinical improvement (hair growth and behavioural change) after HCG injection.
Conclusion
Endocrine diseases can lead to dermatological alterations such as alopecia with or without pruritus. These diseases are much less frequent than parasitic or infectious diseases as a
cause of skin lesions, hence the importance of ruling out these diseases before investigating endocrine causes.
Jana Liebscher, Dr Jutta Hein