In veterinary medicine, too, non-invasive diagnostic and therapeutic approaches have become increasingly important in recent years. That is why biomarkers are more and more being taken into account in the diagnosis. Biomarkers are measurable compounds which can serve as indicators for various physiological or pathological processes and thus have prognostic or diagnostic significance. In some cases, biomarkers can provide important additional information for a complete clinical examination and thereby contribute to medical decision-making. Molecular biomarkers can be determined in biological samples (such as serum, plasma or cerebrospinal fluid) and may be specific molecules, enzymes or hormones.
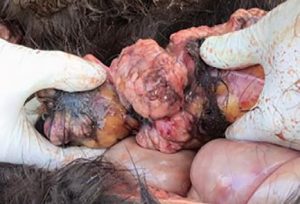
Especially in tumours diagnostics, biomarkers are used as helpful and non-invasive tools for diagnosis, prognosis and treatment monitoring. In equine medicine, tumour diagnostics is becoming more and more important due to the increasing age of the horse population and is still challenging for the practitioner because of the limited application of imaging techniques in horses (thorax, abdomen). Furthermore, when suffering from neoplastic diseases, equine patients tend to show rather non-specific symptoms such as fever, cachexia or mild anaemia, the white blood count often varies only slightly and paraneoplastic syndromes (such as hypercalcaemia) are rarely present. This can make it extremely difficult to find a diagnosis. Equine lymphoma (lymphosarcoma) is the most common malignant tumour disease in horses and can manifest itself in gastrointestinal, cutaneous, mediastinal or multicentric form (Fig. 1).
Depending on the stage of the disease and the site of the tumour, sampling for histopathological diagnosis is often not possible.
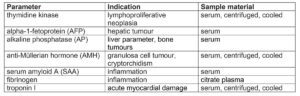
Recently, thymidine kinase has started to be used as a proliferation marker in the diagnosis of equine tumours. It is a cellular enzyme that plays a decisive role during DNA synthesis when the nucleoside thymidine is incorporated into the DNA, which is why its concentration in serum correlates positively with the cell division rate. Since malignant diseases of the haematopoietic and lymphatic system (lymphoma, leukaemia, multiple myeloma) are often highly proliferative, thymidine kinase can be determined as a so-called proliferation marker. Severe inflammation can lead to slight increases in thymidine kinase and must be taken into account in the differential diagnosis. In this case, acute-phase proteins should additionally be determined. Furthermore, a negative result does not rule out an underlying tumoural disease.
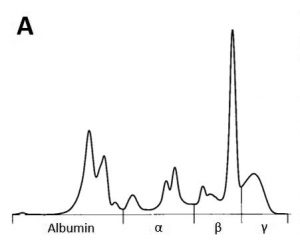
Serum protein electrophoresis can be an additional diagnostic and very cost-efficient option in case of suspected tumours. Monoclonal changes, often in the beta or gamma fraction, are the result of excessive production of a specific immunoglobulin by a plasma cell clone and have mainly been described in lymphoproliferative diseases (Fig. 2 A and B).
If hepatic tumours are suspected (e.g. hepatocellular carcinoma), alpha-1- fetoprotein (AFP) can be determined. AFP is a glycoprotein that is physiologically formed in perinatal foals (up to 1.5 years of age) and is, thus, also elevated in pregnant animals. In adult and non-pregnant horses, an increase may indicate a liver tumour, as AFP is produced by the tumoural hepatic cells and elevated serum levels can then be measured. Alkaline phosphatase (AP) is used less often in the diagnosis of liver tumours. Increased enzyme activities of AP in serum are more likely to be found in bone tumours (e.g. osteosarcoma), although these are rather rare in horses.
- Fig. 1: Post-mortem findings in a 3-year-old Icelandic mare. The horse had a clinical historyof fever, epistaxis and reduced general condition. Laboratory diagnosis showed severe anaemia (erythrocytes 1.55 T/l, haematocrit 0.08 l/l, haemoglobin 54 g/l) with thrombocytopenia (17 G/l). Histopathologically, lymphoma could be confirmed.
- Fig. 2A: Serum protein electrophoresis curve with monoclonal peak in the beta fraction.
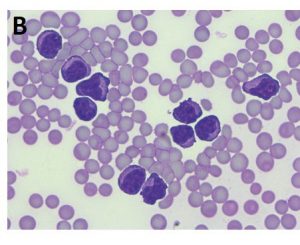
- Fig. 2B: This horse showed severe leukocytosis including numerous atypical lymphocytes in the peripheral blood, a significantly elevatedserum amyloid A concentration (850.15 μg/ml) and a serum thymidine kinase level of 19.3 U/l (reference range < 3 U/l)
A quite common type of tumour in mares is granulosa cell tumour (GCT). A diagnosis can often be conclusively made by determining the anti-Müllerian hormone (AMH). AMH is a glycoprotein that influences sexual differentiation during embryonic development and is formed by granulosa cells during folliculogenesis in mature females and by Sertoli cells in testicular tissue in males. Anti-Müllerian hormone can therefore be used as a laboratory diagnostic marker, not only in the diagnosis of tumours but also for various other clinical issues.
For the diagnosis of granulosa cell tumours, AMH is the most sensitive diagnostic marker (98%) compared to inhibin (80 – 90%) and testosterone (50%). Typically, these mares show clinical signs of anoestrus, very low progesterone levels (< 1ng/ml) and a unilaterally enlarged ovary with a striking honeycomb-like appearance on ultrasound. The contralateral ovary, on the other hand, is usually greatly reduced in size. Often, sonographic differentiation from other ovarian diseases, such as ovarian haematoma, teratoma or cystadenoma, is not clearly possible. The determination of AMH can be helpful here, as it is produced in large quantities by the granulosa cells. If the determination of AMH does not provide a reliable result (e.g. if a granulosa cell tumour is beginning to develop), retesting is recommended after 3 – 4 months at the earliest. Furthermore, diagnosis of a GCT by determining AMH is also possible during pregnancy, as the serum AMH level, unlike testosterone or inhibin, remains unaffected in late pregnancy
In the normal cycle of the mare, too, AMH is not subject to any significant fluctuations and, in contrast to testosterone (adrenal cortex), is only produced in the ovary itself. In older mares (> 20 years), a decline in AMH level may occur correlating with a decrease in follicular reserve (primordial follicles).
Determination of AMH can also be useful in stallions. Unlike oestrone sulphate, AMH concentration in male animals is conclusive for the diagnosis of cryptorchidism regardless of age and thus represents a useful biomarker for the presence of testicular tissue. The baseline testosterone level in cryptorchid stallions can be low or borderline and only provides little information when determined solely. Testosterone should therefore always be measured while performing an hCG stimulation test (baseline and stimulation value). Due to its rather long half-life (1.5 – 2 days), AMH should only be determined after a minimum of 2 weeks post castrationem, since serum concentrations will then have decreased to a diagnostically conclusive level. Before these 14 days have passed, testosterone is more suitable as it has a much shorter half-life (approx. 1 h). Very low AMH levels in intact stallions may indicate testicular degeneration or are due to the season (autumn/winter). Age-related testicular degeneration is more common in stallions at an advanced age, but AMH levels do not provide information on the fertility of an animal. Very high levels, on the other hand, in combination with clinical signs, can be an indicator of Sertoli cell tumour and should always be clarified by histopathological examination.
Yet biomarkers do not only play a role in the diagnosis of tumours. If inflammation is suspected, acute-phase proteins such as serum amyloid A (SAA) or fibrinogen may also be helpful to assess the clinically non-visible severity of the inflammation or to estimate therapeutic success.
In general, acute-phase proteins are divided into negative ones, whose concentration in blood decreases during the acute-phase response (e.g. albumin), and positive acute-phase proteins, which, in turn, increase during inflammation. Positive acute-phase proteins are either major (rapid increase within a short period of time by 10- to 1000-fold), moderate (slow increase by 5- to 10-fold with a plateau phase) or minor acute-phase proteins (0.5- to 5-fold increase). The formation of acute-phase proteins is initiated during the acute-phase response of inflammation. This is an early, non-specific systemic reaction to tissue damage from infection (bacterial, viral or parasitic), trauma or neoplasia. In addition to local and systemic effects, the release of inflammatory mediators, such as pro-inflammatory cytokines, stimulates the synthesis of acute-phase proteins in the hepatocytes of the liver.
In horses, determination of SAA as major acute-phase protein is increasingly used in clinical laboratory diagnostics. SAA is an apolipoprotein which, on the one hand, chemotactically recruits inflammatory cells into an inflamed area, but on the other hand suppresses lymphocyte proliferation. In healthy horses, serum SAA can only be detected in very low concentrations. If a noxious agent is present, there is a very rapid (within 6 – 12 h) and strong increase in concentration (100- to 1000-fold) and also a very rapid decrease when the noxious agent is removed (within 12 h). SAA is a very sensitive marker of early inflammatory processes, which can provide important assistance in diagnosis, prognosis and monitoring. Especially when quick action is essential, as for example in foal medicine, SAA levels can be measured to detect septicaemia, bacterial pneumonia or septic arthritis at an early stage. Serum levels of SAA can also help to differentiate between bacterial and viral infections, but should always be interpreted in the context of the general clinical examination and other laboratory parameters, as SAA is a non-specific acute-phase protein. Slightly elevated levels can be measured after stress, transport, vaccination or heavy exertion. Furthermore, increased SAA should never be the only criterion for antibiotic treatment. The white blood count, clinical chemistry and other markers of inflammation and, of course, clinical and bacteriological examinations should also be included.
Fibrinogen, which is a moderate acute-phase protein, is also used as a diagnostic tool in equine medicine. Compared to SAA, it only increases by 1- to 2-fold within the first 24 h after the noxious agent and peaks after approx. 48 h. Fibrinogen may remain elevated for a few weeks and is therefore not as sensitive when monitoring. Fibrinogen can only be determined from citrate plasma.
A quick and early diagnosis is not only relevant in acute inflammation, but also for organs with a low regeneration rate such as the cardiac muscle. A useful biomarker for acute myocardial necrosis is cardiac troponin I, which can be used as a specific cardiac parameter in horses. Cardiac troponins are proteins that play a role in regulating the myocardial contraction and relaxation. If cells are damaged, increased serum levels of cardiac troponin can be detected after approx. 5 – 7 h. Causes may be viral or bacterial, congenital heart diseases, deficiencies, toxins or neoplasia as well.
So overall, several different, very sensitive biomarkers are available in clinical laboratory diagnostics. Separately or in combination, they can provide suitable assistance in the practice in terms of diagnosis, assessment of prognosis and to predict therapeutic success as well as to support decisions on treatment.
Svenja Möller