Parvoviruses play a significant role as infectious agents in various species. They are small, nonenveloped viruses with a linear, single-stranded DNA genome. They are very stable in the environment and can lead to high animal losses, especially in larger facilities such as animal shelters and breeding kennels (Muzyczka and Berns, 2001; Decaro and Buonavoglia, 2012).
According to the current state of knowledge, the family Parvoviridae comprises three subfamilies, with the subfamily Parvovirinae covering the genera which are important for vertebrates. Within this subfamily, there are currently 10 genera: Amdoparvovirus, Artiparvovirus, Aveparvovirus, Bocaparvovirus, Copiparvovirus, Dependoparvovirus, Erythroparvovirus, Loriparvovirus, Protoparvovirus and Tetraparvovirus.
The viruses that are most relevant for dogs and cats are found in the genera Bocaparvovirus and Protoparvovirus (ICTV, 2022). Some important parvoviruses and their clinical pictures in dogs and cats are presented below.
Canine parvoviruses
Protoparvovirus in dogs
The best-known protoparvovirus is canine parvovirus 2 (CPV-2), which was identified in the 1970s as the main cause of viral enteritis in dogs (Cooper et al., 1979). The virus became endemic worldwide and several virus strains are now known to cause disease, especially in young dogs (Hoelzer and Parrish, 2010; Decaro et al., 2020).
-
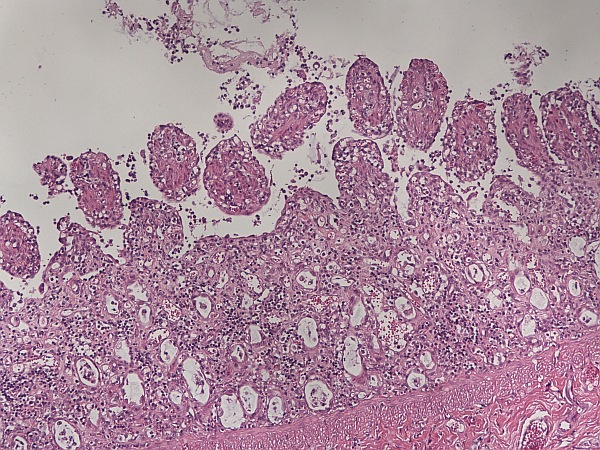
Fig. 1: Small intestine: marked villous atrophy and fusion with necrosis of the crypt epithelium (HE stain)
Photo credits: Laboklin
-
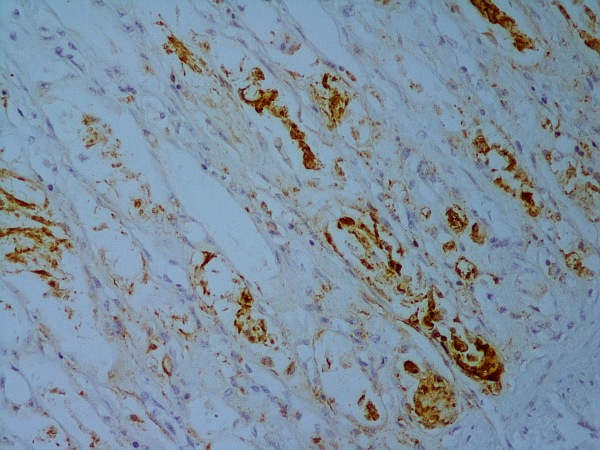
Fig. 2: Small intestine: immunohistochemical detection of parvovirus antigen
Photo credits: Laboklin
Pathogenesis
Puppies of immune female dogs are usually protected from parvovirus infection for about 2 to 3 months through the uptake of maternal antibodies in colostrum. If vaccination is given too early, it can be neutralised by maternal antibodies, so the timing of vaccination and the vaccination schedule used are important (Decaro et al., 2020). CPV-2 is taken up via the faecal-oral route and the first clinical signs usually appear after an incubation period of 4 – 14 days. After ingestion, the virus first replicates in the local lymphoid tissue of the oropharynx. This is followed by viraemia (Sykes, 2014). Via the transferrin receptor, CPV-2 enters cells with a high division rate (Parker et al., 2001), such as those particularly found in the intestine (intestinal crypts) and in lymphoid organs like thymus, lymph nodes and bone marrow (Sykes, 2014; Mylonakis et al., 2016).
Pathological changes
Histologically, classic lesions such as necrosis of crypt epithelium, the shortening of villi and villous atrophy (Figure 1), giant cell formation as a sign of crypt regeneration and lymphatic depletions are found. In some cases, intranuclear viral inclusion bodies can also be seen (Carman and Povey, 1985; Decaro and Buonavoglia, 2012, Osterhaus et al., 1980).
Clinical picture
Clinically, canine parvovirus infection is characterised by gastroenteritis with haemorrhagic diarrhoea and vomiting. In addition, fever and anorexia often occur as well as dehydration, which can lead to death. Moreover, depletion of lymphoid tissue may facilitate secondary systemic bacteraemia, which can be fatal. Furthermore, lymphopenia is often observed as a result of direct lymphocytolysis (Mazzaferro, 2020). Myocarditis is another clinical picture and mainly occurs in very young dogs (Hayes et al., 1979).
Detection, differential diagnoses and treatment
In suspected cases, diagnosis of a parvovirus infection can be made by detecting virus particles in faeces or swabs. This can be done in various ways, especially by ELISA and PCR, or with methods that are more commonly used in specialised, often researchoriented laboratories, such as electron microscopy, haemagglutination and virus isolation. Immunohistochemical testing for parvovirus antigen is also possible in some laboratories (Figure 2). PCR is a very sensitive and specific detection method, while ELISA is often already used in the clinic (Mazzaferro, 2020). For parvovirus detection by PCR, faeces, EDTA blood or tissue can be submitted. After vaccination with live vaccine, PCR can be positive for up to 4 weeks. For parvovirus antigen determination, faeces can be sent in. Here, a positive result is possible 5 – 12 days after vaccination with live vaccine.
For differential diagnosis, particularly other viruses, bacteria, endoparasites, but also food intolerances or feeding-associated factors, intoxication, foreign bodies, pancreatitis, hypoadrenocorticism or IBD (inflammatory bowel disease) should be considered (Sykes, 2014).
In terms of therapy, symptomatic treatment is recommended. In severe courses of disease due to secondary bacteraemia, therapy should be combined with antibiotic treatment. Analgesia is sometimes recommended as well (Mazzaferro, 2020). The most important preventive measure against canine parvovirus infection is vaccination (Decaro et al., 2020). Furthermore, effective disinfectants must be used after outbreaks and infected animals must be isolated from healthy ones (Sykes, 2014).
In addition to the rather “typical” and wellknown presentation, CPV-2 has also been described in association with other clinical pictures. These include myocarditis, hepatitis, chronic immune complex disease and meningoencephalitis (Berns and Parrish, 2007). Since canine parvoviruses and feline panleukopenia virus have repeatedly been detected in neurons, possible replication in neurons has been discussed for some time (Garigliany et al., 2016; Schaudien et al., 2010).
Bocaparvovirus in dogs
The genus Bocaparvovirus also contains clinically relevant parvoviruses, such as the so-called minute virus of canines (formerly canine parvovirus 1). It has been described in the context of respiratory disease in young dogs, abortions and, less often, diarrhoea. In adult animals, infections are usually subclinical (Kapoor et al., 2012; Harrison et al., 1992).
Another pathogen is canine bocavirus 2, which has been isolated from dogs with respiratory diseases, but also from healthy dogs. Furthermore, a strain was described that caused CPV-2-like lesions in a litter of young dogs (Bodewes, 2014).
Canine bocavirus 3 was found in the liver of a dog that was also infected with a novel circovirus (Li et al., 2013).
Feline parvoviruses
Protoparvovirus in cats
Feline panleukopenia virus (FPV) is closely related to CPV-2 and has already been previously observed, so evolution of CPV-2 from FPV with host range adaptation is being discussed (Mazzaferro, 2020).
Similar to CPV-2, the first viral replication takes place in the oropharynx within 18 – 24 hours after oral or intranasal infection. Viraemia and distribution of the viruses in the organism occurs within 2 days up to a week. FPV also infects cells with a high division rate and is therefore associated with similar signs and lesions as CPV-2 (Stuetzer and Hartmann, 2014). Moreover, foetal or neonatal infections lead to defects in the central nervous system. They result from infection of the neuroblasts of the external granular cell layer during the development of the cerebellum, which occurs in the late gestational and early neonatal period. These changes can then lead to cerebellar hypoplasia (Aeffner et al., 2006; Csiza et al., 1971; Garigliany et al., 2016; Poncelet et al., 2013). As with canine parvovirus, FPV has been described in neurons (Garigliany et al., 2016; Schaudien et al., 2010) as well as in a young cat with ataxia. Histologically, neuronal vacuolation was found. In these neurons, FPV-specific nucleic acids and parvovirus antigen were detected (Pfankuche et al., 2020).
Bocaparvovirus in cats
Bocaparvoviruses are also known in cats and, similar to canine bocaparvoviruses, are sometimes isolated from asymptomatic cats. A clear association of the infection with enteric or other systemic diseases has not yet been conclusively clarified (Piewbang et al., 2019).
Conclusion:
Parvoviruses in dogs and cats are very stable pathogens that can cause problems, especially in larger facilities, and continue to play a major role in veterinary medicine. More variants are regularly being discovered, too. In addition to the “classic” gastrointestinal signs, parvoviruses have also been described in association with other clinical pictures such as myocarditis, hepatitis, respiratory diseases, immune complex diseases, abortions and CNS disorders. However, their significance has not yet been conclusively clarified for all clinical presentations and further research is necessary. Nonetheless, parvovirus infections should also be considered in the differential diagnosis of these clinical pictures.
Author: Dr Ph.D. Vanessa Nippold
References:
-
Aeffner, F, Ulrich, R, Schulze-Ruckamp, L. Cerebellar hypoplasia in three sibling cats after intrauterine or early postnatal parvovirus infection. Dtsch Tierarztl Wochenschr. 2006;113(11):403–406.
-
Berns, K, Parrish, CR. Parvoviridae, in: Knipe DM, Howley PM. Fields Virology, 5th ed., 2007. pp. 2437–2477.
-
Carman, PS, Povey, RC. Pathogenesis of canine parvovirus-2 in dogs: histopathology and antigen identification in tissues. Res. Vet. Sci. 1985;38:141–150.
-
Cooper, BJ, Carmichael, LE, Appel, MJ, Greisen H. Canine viral enteritis. II. Morphologic lesions in naturally occurring parvovirus infection. Cornell Vet. 1979;69(3):134-144.
-
Csiza, CK, De Lahunta, A, Scott, FW. Pathogenesis of feline panleukopenia virus in susceptible newborn kittens II: pathology and Immunofluorescence. Infect Immun. 1971;3(6):838–846.
-
Decaro, N, Buonavoglia, C, Barrs, VC. Canine parvovirus vaccination and immunisation failures: Are we far from disease eradication? Vet Microbiol. 2020;247:108760.
-
Decaro, N, Buonavoglia, C. Canine parvovirus—a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012;155:1–12.
-
Garigliany, M, Gilliaux, G, Jolly, S. Feline panleukopenia virus in cerebral neurons of young and adult cats. BMC Vet Res. 2016;12:28.
-
Harrison, LR, Styer, EL, Pursell, AR, Carmichael LE, Nietfeld, JC. Fatal Disease in Nursing Puppies Associated with Minute Virus of Canines. J Vet Diagn Invest. 1992;4(1):19-22.
-
Hayes, MA, Russell, RG, Babiuk, LA. Sudden death in young dogs with myocarditis caused by parvovirus. J Am Vet Med Assoc. 1979; 174(11):1197-1203.
-
Hoelzer, K, Parrish, CR. The emergence of parvoviruses of carnivores. Vet Res. 2010;41(6): 39. International Committee on Taxonomy of Viruses, ICTV, 2022.
-
Kapoor, A, Mehta, N, Dubovi, EJ, Simmonds, P, Govindasamy, L, Medina, JL, Street, C, Shields, S, Lipkin, WI. Characterization of novel canine bocaviruses and their association with respiratory disease. J Gen Virol. 2012; 93(2):341–346.
-
Li, L, Pesavento, PA, Leutenegger, CM, Estrada, M, Coffey, LL, Naccache, SN, Samayoa, E, Chiu, C, Qiu, J, Wang, C, Deng, X, Delwart, E. A novel bocavirus in canine liver. Virol. J. 2013;10:54. Mazzaferro, EM. Update on Canine Parvoviral Enteritis. Vet Clin North Am Small Anim Pract. 2020; 50(6):1307–1325.
-
Muzyczka, N, Berns KI. Parvoviridae: The viruses and their replication, in: Knipe DM, Howley PM. Fields Virology, 4th ed. 2001. pp. 2327-2359
-
Mylonakis, ME, Kalli, I, Rallis, TS. Canine parvoviral enteritis: an update on the clinical diagnosis, treatment, and prevention. Vet Med (Auckl). 2016;7: 91–100.
-
Osterhaus, AD, Drost, GA, Wirahadiredja, RM, van den Ingh, TS. Canine viral enteritis: prevalence of parvo-, corona- and rotavirus infections in dogs in the Netherlands. Vet. Q. 1980;2:181–190.
-
Parker, JSL, Murphy, WJ, Wang, D, O‘Brien, SJ, Parrish, CR. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J Virol. 2001; 75(8): 3896–3902.
-
Pfankuche, VM, Jo, WK, van der Vries, E, Jungwirth, N, Lorenzen, S, Osterhaus, ADME, Baumgärtner, W, Puff, C. Neuronal vacuolization in feline panleukopenia virus infection. Vet Path. 2018;55(2)294-297.
-
Piewbang, C, Kasantikul, T, Pringproa, K, Techangamsuwan, S. Feline bocavirus-1 associated with outbreaks of hemorrhagic enteritis in household cats: potential first evidence of a pathological role, viral tropism and natural genetic recombination. Scientific Reports. 2019;9.
-
Poncelet, L, Heraud, C, Springinsfeld, M. Identification of feline panleukopenia virus proteins expressed in Purkinje cell nuclei of cats with cerebellar hypoplasia. Vet J. 2013;196(3):381–387.
-
Schaudien, D, Polizopouloi, Z, Koutinasm A. Leukoencephalopathy associated with parvovirus infection in Cretan Hound puppies. J Clin Microbiol. 2010;48(9):3169-3175.
-
Stuetzer, B, Hartmann, K. Feline parvovirus infection and associated diseases. Vet J.2014;201(2):150-155.
-
Sykes JE. Canine Parvovirus Infections and Other Viral Enteritides. Canine and Feline Infectious Diseases. 2014;141–151.