WNV was first isolated in December 1937, from a 37-year-old, febrile woman in the West Nile district in the Northern Province of Uganda. In Europe, human cases were documented in the 1960’s. Currently, West Nile Virus is present on all continents. The West Nile virus belongs to the Flaviviridae family, like the dengue virus, the TBE virus and the Usutu virus. It is transmitted by various mosquitoes.
Number of cases
In Europe, most human and animal cases occur in Italy, Greece, Serbia and Romania. The cases reported to the European Centre for Disease Control (ECDC) for 2022 are shown in Table 1.
Table 1: WNV case numbers for the year 2022; Source: ECDC
| Cases Human | Deaths Human | Cases Horse/ Bird | |
| Italy | 586 | 37 | 47/258 |
| Greece | 284 | 31 | 9 / – |
| Serbia | 226 | 12 | – |
| Romania | 46 | 5 | – |
| Germany | 11 | – | 16 / 51 |
| Austria | 6 | – | 1 / 2 |
| France | 4 | – | 6 / – |
| Spain | 5 | – | 8 / 9 |
| Hungary | 14 | – | 3 / 1 |
| Portugal | 0 | – | 3 / – |
| Croatia | 8 | – | – / 2 |
| Slovakia | 1 | – | – |
-
Fig. 1: Culex mosquitoes are considered the most important vectors for WNV transmission
Image source: www.cdc.gov/mosquitoes
-
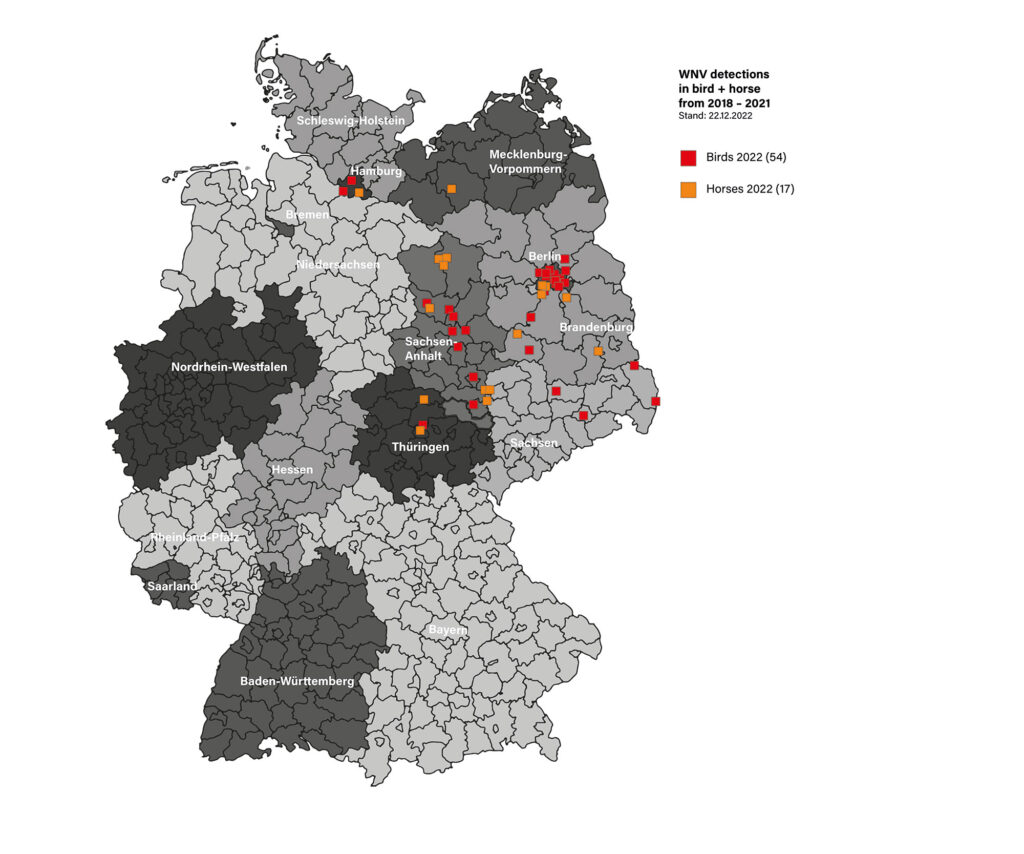
Fig. 2: Geographical distribution of reported WNV cases in horses and birds in Germany
Image source: Laboklin
-
Fig. 3: Mosquito larvae of Culex sp. can develop into an imago in a few days in midsummer
Image source: Dr Regina Wagner
Pathogenic biology
WNV is a vector-borne virus of with wild birds being the main reservoir. These wild birds, often remain asymptomatic. However, corvids, passerine birds as well as certain birds of prey and owls can become severely ill and succumb to the virus. The virus is transmitted by ornithophilic mosquitoes, mainly Culex sp. (Figure 1). Aedes sp. and others such as the tiger mosquito can also be carriers. Humans and horses are considered dead-end hosts because the viral load during an infection is too low to infect other animals. Infections however, also been described in dogs, cats and sheep.
As a rule, arthropods (mosquitoes) are necessary for transmission. Individual cases of direct transmission through blood transfusion, organ transplantation, intrauterine transmission as well as person to person have been described.
Clinical image
WNV-associated diseases occur seasonally, from early summer into autumn, depending on the activity of the mosquitoes. As a rule, usually individual horses are affected. The regional distribution is also related to the flight routes of migratory birds (Figure 2).
In humans, about 80 % of infections remain asymptomatic, with febrile general illness (West Nile fever) developing in about 20 % of cases.
Less than 1 % of human cases (1 in 150) develop WNV-associated meningoencephalitis, with severe infections, this is more prevalent in the elderly or immunocompromised patients.
Most horses also experience asymptomatic seroconversion. Only about 10 – 20 % of horses develop clinical signs after an incubation period of 3 – 15 days, of which only about 8 % are severe neurological signs. Only a few have fever as a characteristic clinical sign. Non-specific clinical signs can be: anorexia, mild fever, somnolence, colic or lameness. When meningoencephalitis develops, there are non-specific neurological signs that may be asymmetrical and progressive. Stumbling, hindlimb paralysis, head resting, dysphagia, ataxias, muscle tremors or weakness and even recumbency have been documented. Up to 40% of horses presenting with neurological signs die or have to be euthanised. Permanent damage can remain after the infection has been cured.
Diagnostic
Serology
In horses, different ELISA tests are available for the detection of IgG and IgM antibodies. IgM antibodies rise a few days post infection and remain elevated for up to 4 – 6 weeks.
CAVE: IgM antibodies can be detected up to 52 days after a recent vaccination, therefore the vaccination status should be considered when assessing laboratory findings. IgG antibodies rise somewhat later and may persist longer (about 1 year). Both IgG and IgM can lead to cross-reactions with other flaviviruses (e.g. TBE virus and Usutu virus). Therefore, suspected cases must be confirmed by several virus neutralisation tests (VNT) to differentiate the flavivirus-specific antibodies of the national reference laboratory (FLI, AGES).
Direct virus detection by means of RT-PCR
Initially, there is a short viraemia, which has often already subsided by the time the symptoms appear, so that a PCR from EDTA blood has only a very low sensitivity for diagnosis. RT-PCR from cerebrospinal fluid or brain tissue is conclusive in a positive case, but a negative PCR from cerebrospinal fluid does not exclude the infection, as the virus can also be restricted to the nerve tissue.
On post-mortem, very high levels of virus can be detected in the CNS. The use of Personal protection equipment (PPE) is important when collecting such samples. There is a case described where WNV was transmitted to a veterinary student during the dissection of a horse (removal of the brain).
Differential diagnoses
Other neurotropic infections can be considered as differential diagnoses. In particular, equine herpesvirus myeloencephalopathy due to EHV-1 or EHV-4 should be clarified in case of neurological symptoms (PCR from EDTA blood and from respiratory swabs without medium). TBE (serology IgG and IgM from serum or IgG from CSF) and Borna (PCR and/or antibodies from blood or CSF) are also possible differential diagnoses. In addition, other causes of neurological symptoms, such as injuries or poisoning, should be excluded (e.g. tetanus, botulism, listeriosis, leptospirosis).
Duty of disclosure
Infection with WNV is a zoonotic disease that must be monitored in the EU and Switzerland.
In Germany, acute infection in horses is notifiable. The basis for this is a positive IgM ELISA. Confirmation of acute infection must be carried out by the national reference laboratory due to cross-reactions of flaviviruses via VNTs. A positive IgG antibody test alone is not a basis for mandatory notification. IgG antibodies can originate from a vaccination or a longer-standing (possibly asymptomatic) infection.
Cross-reactivity must also be considered. In routine diagnostics, there is currently no ELISA test that can distinguish between antibodies against WNV from the vaccines available on the market and field virus antibodies. In Austria, notification is mandatory for all equine encephalomyelitis, regardless of its origin.
Prophylaxis
Early symptomatic treatment can significantly improve the survival rate of affected horses.
Since a causal therapy is not possible, prophylactic measures come to the fore. Various vaccines are approved for the use in horses.
Vaccination is recommended especially in areas where cases have already occurred in humans or animals and for horses competing abroad.
The basic immunisation should be completed before the start of the mosquito season. Another component of prophylaxis is to ensure protection against mosquitoes. Here, the use of repellents or protective nets/blankets should be mentioned. In addition, special attention should be paid to possible breeding sites for mosquitoes. Standing water accumulations are problematic, because in summer temperatures mosquitoes can develop from egg to larvae to imago within a few days (Figure 3). Vessels with standing water/water accumulations should be covered mosquito-proof or regularly cleaned and freshly filled (e.g. watering cans, water troughs, rainwater containers). Puddles of water, e.g. in lying car tyres or in folds of foil, should also be avoided.
Eva Kahnt