Human coeliac disease is a gluten-induced enteropathy characterised by a specific genetic genotype (HLA-DQ2/HLA-DQ8 genes) and the detection of autoantibodies against gluten (Leonard et al. 2017).
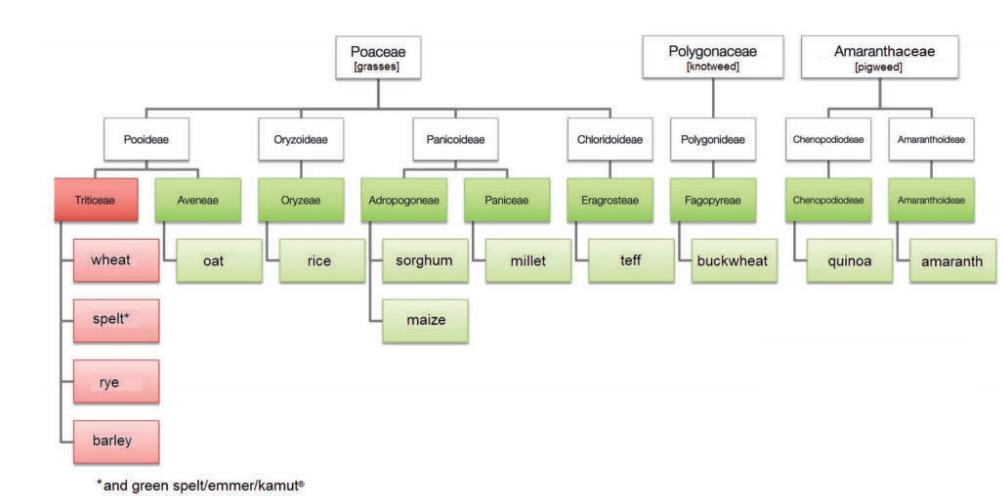
Gluten is the main storage protein of grains (wheat, rye, barley, spelt; Fig. 1) and a complex mixture of hundreds of similar yet slightly different proteins, especially gliadin and glutenin (Biesiekierski 2017).
In humans, the clinical picture of coeliac disease was first described by Samuel Gee in 1887, although wheat was not identified as a possible trigger until about 60 years later, in 1941, by William Diecke (Diecke 1941).
Clinically, those affected mainly suffer from malabsorption, diarrhoea and children mostly from growth disorders (Andersen 1947). Prevalence in the total population is about 1%, with regional differences (Husby et al. 2012).
Two clinical pictures have been described that are associated with gluten intolerance in dogs. One is a symptom complex in Irish Setters with mainly digestive disorders, and the other is a clinical picture in Border Terriers mostly characterised by seizures.
Recently, however, mixed forms of the clinical pictures have also received greater scientific attention (Lowrie 2017).
In connection with gluten intake, digestive disorders with inappetence, chronic diarrhoea and weight loss as well as growth retardation in young animals have been described in Irish Setters similar to coeliac disease in humans. Usually, the onset of clinical signs is at the age of six months (Daminet 1996). Pathologically, increased intestinal permeability, partial villous atrophy and intraepithelial infiltration with lymphocytes can be detected. However, mucosal damage is typically less pronounced in Irish Setters than in people suffering from coeliac disease (Pemberton et al. 1997).
This gluten sensitivity in dogs, which is associated with digestive problems, appears to be breed-specific to the Irish Setter (Daminet 1996) and, according to Garden et al. (2000), is inherited in an autosomal recessive manner.
After changing to a gluten-free diet, all clinical signs usually improve significantly and also immediately (Pemberton et al. 1997).
Although elevated gliadin IgG antibody titres are detectable in people with coeliac disease and are also used as a screening test (Leonard et al. 2017), a first study by Hall et al. from 1992 did not measure any elevated gliadin IgG antibody titres in Irish Setters with gluten-sensitive enteropathy. Hall et al. (1992) speculated that immune complex binding may be a possible explanation for this. However, this connection, which so far has only been investigated in the study mentioned above, is questionable and should be examined more closely in further studies. This is because the test for the presence of antibodies could also be a valuable diagnostic tool for gluten-induced enteropathy in Irish Setters (manuscript in preparation).
-
Fig. 1: Taxonomy of gluten-containing and gluten-free grains.
Photo credits: Diana Studerus
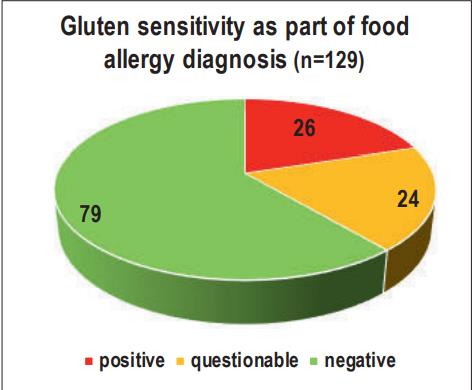
- Fig. 2: Gluten sensitivity as part of food allergy diagnosis (n=129)
In Border Terriers, however, canine epileptoid cramping syndrome has been described as being linked to the intake of gluten-containing food (Black et al. 2014). According to the latest publications, this clinical picture – also known as “Spike’s disease” – should rather be called paroxysmal gluten-sensitive dyskinesia (PGSD), as it is not associated with epileptiform seizures and should, in fact, be clearly distinguished from them (Lowrie 2017).
It involves abnormal movements that only occur episodically, are completely self-limiting and during which the animals, unlike epileptiform seizures, are fully conscious all the time (Lowrie 2017). Abnormal movements described include difficulty walking, mild tremor, convulsions and dystonia (involuntary muscle contractions) (Black et al. 2014).
Typically, all four limbs were affected as well as the head and the neck. In between these phases of abnormal movements, which can last from 2 to 30 minutes, there are often long periods of absolutely normal behaviour (Black et al. 2014).
Staring vacantly into space (while fully conscious), gastrointestinal symptoms and atopy with severe pruritus have also been described (Lowrie 2017).
Clinical signs are often seen before the age of three years, but feeding a gluten-free diet leads to an immediate reductionof these signs in 50% of the cases (Black et al. 2014).
According to Lowrie (2017), Border Terriers are the only breed in which PGSD has been proven for sure. However, Park et al. (2014) also described a case of PGSD in a nine-month-old Yorkshire Terrier.
Elevated levels of modified gliadin peptide IgG (MGP-IgG) and tissue transglutaminase IgA (TG-2-IgA) antibodies provide a specific marker for the diagnosis of paroxysmal gluten-sensitive dyskinesia (PGSD) in Border Terriers.
With a gluten-free diet (at least 3 – 9 months), both antibody titres decrease (Lowrie et al. 2015). This can be used for therapy monitoring, but may also lead to false negative results if the test is carried out with previously adapted gluten-free feeding.
In an in-house study of 129 dogs that had undergone a food allergy test, we were able to detect a positive or questionable result regarding a possible gluten sensitivity in 26 and 24/129 cases, respectively. It is interesting to note that mainly mongrels (n=10), French Bulldogs (n=5), German Shepherds (n=4) and Labrador Retrievers (n=4) were affected.
Conclusion
Even though there are certain similarities between human coeliac disease and gluten-sensitive enteropathy in Irish Setters, gluten sensitivity in Border Terriers manifests itself quite differently as paroxysmal gluten-sensitive dyskinesia. Initial publications and in-house studies indicate that not only Irish Setters and Border Terriers, but also other breeds react to the intake of gluten-containing food with clinical signs and positive antibody titres.
Dr. Julia Grassinger
Test for canine gluten sensitivity:
- at least 0.5 ml of serum
- determination of MGP-IgG and TG-2-IgA antibodies
- available for all dog breeds