How would we diagnose without laboratory tests?
Whether it is blood count, pathogen detection or pathology – successful diagnostics without laboratory tests is hardly imaginable.
What is often not considered is how important it is what happens to the sample before it arrives at the laboratory. We want to make the best of your samples. Help us to do so!
The basics
The clinical history helps us to help you.
The diagnostic task is particularly important, especially with blood smears, cytology, histopathology and pathogen detection by culture. The more we know about the patient and the problem, the more precisely we can look for specific changes and interpret the findings for you.
Accurate laboratory values are obtained from the right material.
If a parameter is determined from the wrong material, it may become useless for diagnosis. The material you need is indicated on the submission form.
Please let us know what kind of sample you have sent. Plasma can be obtained from citrate, EDTA and heparin blood. Urine, plasma/serum and CSF look very similar. Correct labelling helps to avoid errors.
In a modern laboratory, many things are automated.
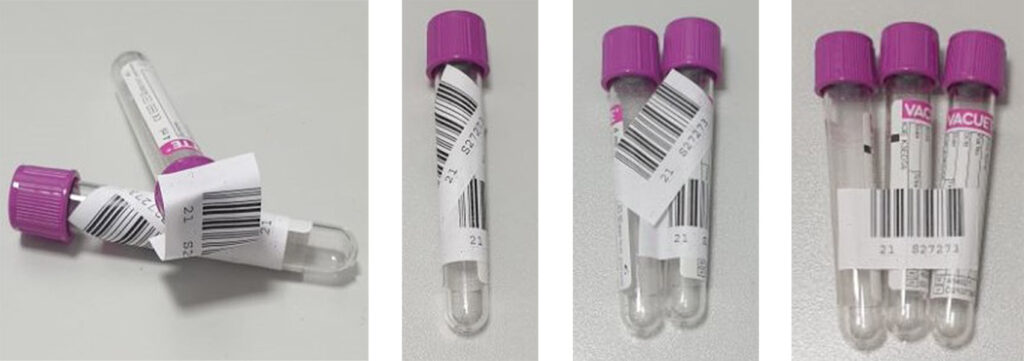
Incorrectly attached or missing barcodes on test tubes lead to additional time and effort. This takes up valuable time in the diagnostic process.
The type of sample packaging can be crucial for analysis and may have consequences for the colleagues in the laboratory.
You should avoid the following: closing syringes with cannulas instead of appropriate plugs, pathology samples in glass containers and faecal samples in gloves. There is a risk of injury, sample contamination and sample loss.
Courier transport is only permitted for correctly packaged and labelled samples.
Use the packaging materials provided (consisting of primary sample tube, secondary transport tube/ container, outer packaging). For liquid samples, there must be absorbent material between the primary and secondary tube/container, and tubes must be protected against breakage. For sending samples to Germany per air-freight, it is absolutely necessary they are packed and marked according to the EU-regulations: all sample tubes are to be packed in leek-proof protective secondary containers and then in rigid cardboard box with extensive cushioning material. The cardboard box should be marked with “UN3373 Biological substance Category B” label.
You can find more details in the Laboklin „Directory of Tests“.
Blood tests
The filling level of the tubes has significant influence on the measured parameters!
If there is too little blood in a tube, there is an excess of anticoagulant. This will, among others, influence cell morphology and lead to incorrectly prolonged coagulation values. Overfilling also causes problems (e.g. coagulation of plasma samples).
The order of the tubes during blood collection can have enormous consequences.
EDTA tubes should be filled last. Even the slightest contamination of the needle with EDTA can lead to significant errors when measuring calcium and potassium. If blood coagulation is needed, the first few drops of blood should either be discarded or another tube should be filled first.
The type of tube affects the laboratory workflow.
The small test tubes with hinged lids are popular. But they pose problems. For one, the lids often do not close completely, so that sample volume gets lost. And for another, the needles of the analysers cannot pierce them. The samples have to be pipetted by hand. This causes delays in the workflow.
The condition of the sample is decisive for how useful it is for diagnosis.
Ideally, the sample is cooled and protected from light until it is shipped. Attention should already be paid in the practice regarding the correct storage of the sample material. A blood sample which has been lying around all day at room temperature in full daylight will yield incorrect results in certain examinations (e.g. bilirubin). The error is in the detail!
Centrifugation and pipetting are tedious but necessary!
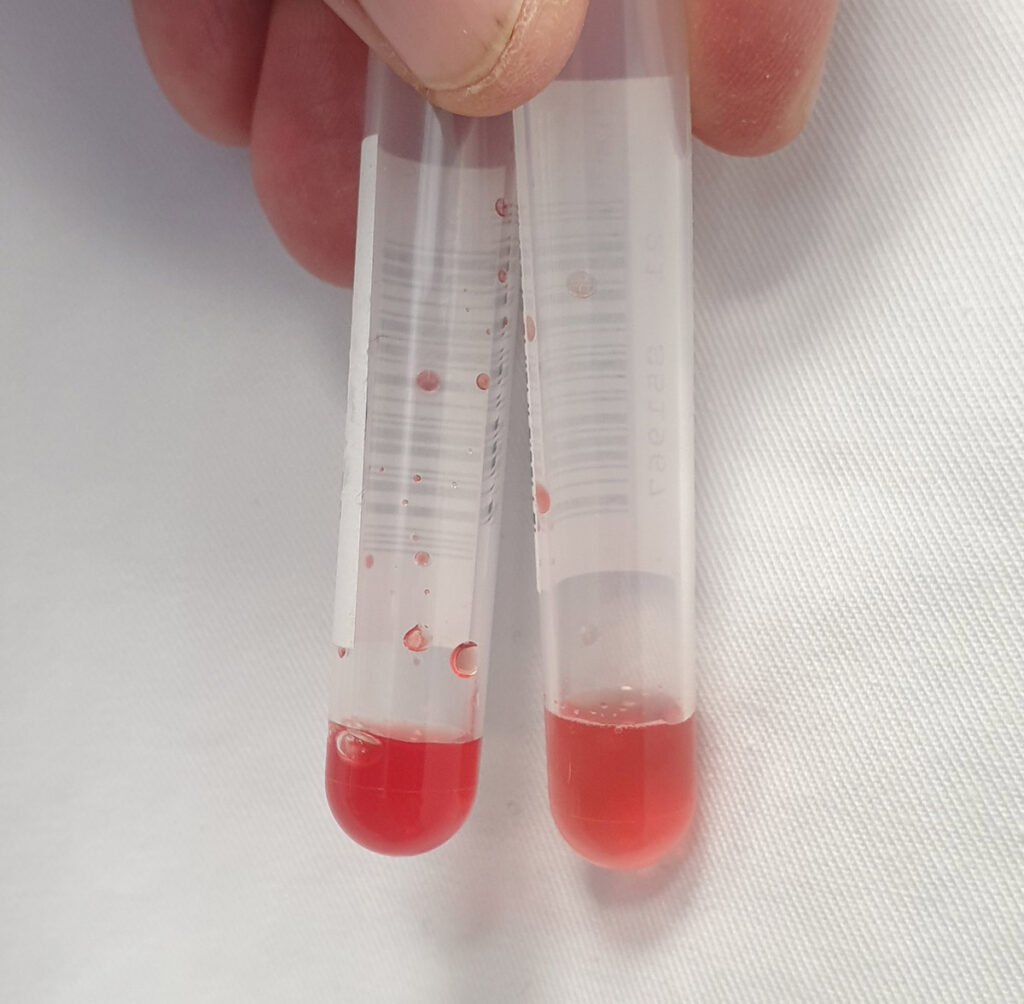
When whole blood is supplied, haemolysis and cellular metabolism will occur. This leads to incorrect measurements, possibly with far-reaching consequences for clinical decisions. Place the sample upright (do not transport it in a lying position at this point!) and separate the serum/plasma as soon as possible after collection (first allow serum to clot at room temperature). Make sure to also pipette off the supernatant for shipping. Merely centrifuging without transferring the supernatant is not helpful!
A blood smear is part of a blood count.
Even the short time of transport leads to fundamental changes in the cells. If you already prepare a smear in the practice, you fix the intact cells on the slide and thus give us the opportunity to make a good evaluation.
-
Fig. 1: Incorrectly attached barcodes
Photo credits: Laboklin
-
Fig. 2: Correctly attached barcode
Photo credits: Laboklin
-
Fig. 3: The ideal fill volume differs from one tube to another (see arrows on the two tubes on the right for the mark of the filling level which needs to be observed).
Photo credits: Laboklin
-
Fig. 4: A sample that has not been centrifuged will almost always become haemolytic
Photo credits: Laboklin
-
Fig. 5: Preparation too thick → cells cannot be differentiated
Photo credits: Laboklin
-
Fig. 6: Good smear → evaluation is possible (fungal hyphae)
Photo credits: Laboklin
-
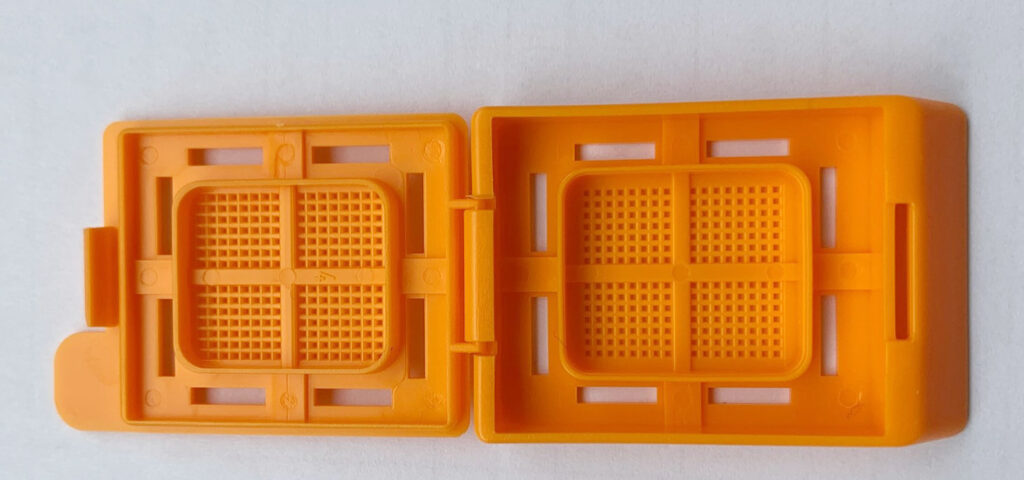
Fig. 7: Embedding cassette
Photo credits: Laboklin
Molecular biology – Pathogen PCR
Pathogen DNA is multiplied by polymerase chain reaction (PCR) and detected by optical methods. This is a direct method of pathogen detection.
However, DNA of dead pathogens is also detected. On the other hand, a negative result does not rule out an infection.
The time at which the sample is taken can be decisive.
It is most likely to detect the pathogen in the blood during viraemia, bacteraemia and parasitaemia or during fever. If pathogens are excreted intermittently in the faeces (e.g. Tritrichomonas foetus), a 3-day pooled faecal sample must be sent in.
Sample material and sample handling need to be considered.
For PCR, EDTA blood is a suitable sample material for pathogen detection from blood. Lithium heparin is a PCR inhibitor and therefore only of limited suitability. For pathogens that are excreted through the mucous membranes (e.g. in respiratory diseases), please use sterile swabs without transport medium. A transport medium may affect the PCR.
Faecal samples should be around the size of a hazelnut. Detection of equine coronavirus is only useful from faeces (mucosal swabs are not suitable). Faeces contain natural PCR inhibitors (e.g. bile acids). If inhibition occurs, we will repeat the test with a diluted sample. This also dilutes the pathogen and unfortunately increases the probability of false negative results.
Depending on the diagnostic task and the signs, other sample materials (e.g. skin biopsies, lymph nodes, urine) could be used, too. These samples are sent in sterile, uncoated test vessels. Fixative solution can destroy DNA and cause false negative results. Samples do not need to be sent cooled but should be stored in the refrigerator until shipment. Repeated freezing and thawing should be avoided.
Please keep in mind that it is not possible to create an antibiogram after doing a PCR test!
Microbiology – Pathogen culture
Taking the sample – what to observe:
Please do not disinfect the changed site before taking the sample; only remove superficial secretions and layers – if present – beforehand with a sterile swab and discard them. Make sure to take as much material as possible and avoid contamination with the physiological flora of the healthy surrounding area.
The right time
To detect pathogens which are capable of multiplying, the sample should be taken as soon as possible after the onset of clinical signs and definitely before the start of antibiotic treatment. If pre-treatment has taken place, it is recommended to take the sample at the earliest one week after administration of the antibiotic was stopped. If treatment is not successful or if the patient worsens during treatment, diagnostics can also be initiated during antibiotic treatment. However, the effect of antibiotic treatment might prevent the pathogen from growing in vitro even if viable bacteria are still present in vivo.
The right site
When selecting the site, it is important to consider where the pathogen is expected.
Depending on the suspected diagnosis, the following sites, for example, should be preferred: for strangles in horses (Sc. equi equi), a deep nasal swab or a guttural pouch lavage sample; in case of pneumonia, a bronchoalveolar lavage is better than a nasal swab; in equine mud fever (Dermatophilus congolensis), skin crusts or an impression smear of fresh lesions with serous fluid on a microscope slide, but not cut hairs; for pyoderma, a swab of the skin lesion or plucked hairs; in case of an abscess, a swab taken from the inside of the abscess capsule is better than directly from the pus; for cystitis, catheter or cystocentesis urine is more conclusive than spontaneous urine, which may be contaminated with bacteria.
Why does the laboratory need to know the sampling site?
Depending on where the sample was taken from, we will use special culture media or adapt the culture conditions (temperature, oxygen, incubation time) to detect typical pathogens. Growing a culture without having background information can result in not detecting important infections.
Very important: The evaluation of the antibiogram according to international guidelines (CLSI) can only be carried out correctly if it is known from where the respective pathogen was isolated. If the site is not specified, we cannot create an antibiogram that can be used according to modern standards!
Cytology
How cytology samples are prepared:
Fluids are spread out like a blood smear, while swabs/cytobrushes are rolled onto the slide. It is best to centrifuge (clear) fluids which are low in cells and then spread the sediment (please indicate “sediment”).
What must be observed to ensure the preparations can be evaluated well:
To avoid autolysis, cytology slides should already be prepared in the practice. Artefacts are reduced if the smear is not too thick and is spread with little pressure. Please always air-dry smears and do not (heat-)fix or cover them.
It is absolutely necessary to ship dry slides in slide mailers so that the cells do not get damaged. Formalin fumes will render the slides useless, so please send histological, formalin-fixed sample material in a separate shipping bag. Cytology samples should not be stored in the refrigerator as water may condense. Last but not least, the clinical history is very important in order to be able to provide a correct and helpful interpretation.
Histopathology
How histology samples are prepared:
Take material from different sites for a representative sample. Avoid necrotic tissue. Tumours should be resected as completely as possible to evaluate the margins. The ideal sample size ranges from 0.4 – 1.0 cm in diameter. Especially with small samples, ensure that there are enough specimens. Avoid artefacts during collection, resulting, for example, from electrocoagulation, disruption and squashing. For formalin fixation, use 10% formalin (= 4% formaldehyde) at a tissue to formalin ratio of 1:10, or better 1:20; do not use any other fixatives (such as alcohol), otherwise, it is better to send the sample unfixed – the latter is usually not problematic if the sample is received the next day. At subzero temperatures, a little added alcohol prevents freezing.
What must be observed to ensure the preparations can be evaluated well:
Please remember to use leak- and break-proof containers for shipping. For very small biopsies, shipping in embedding cassettes can prevent the sample from falling apart. The clinical history helps the pathologist to focus the attention on relevant changes. This is indispensable for a good evaluation!
Dr. Jennifer von Luckner