Allergen-specific immunotherapy (ASIT, hyposensitisation) represents the only causal treatment for allergic diseases in horses. These allergies can manifest as cutaneous signs, including pruritus and urticaria (atopic dermatitis and sweet itch), or as respiratory symptoms, such as equine asthma. In some cases, headshaking may also be associated with allergic reactions. The most common causative allergens are environmental – including pollens, house dust or storage mites, and moulds – as well as insect allergens.
Allergies cannot be cured, but only managed, and require lifelong treatment. ASIT is the only therapeutic option that acts causally on the disease process. It is an effective and safe treatment, with successfully treated horses showing markedly reduced symptoms or even becoming completely symptom-free. By administering an extract containing the relevant allergens, the immunological response to environmental allergens is modulated. The conventional ASIT protocol consists of subcutaneous injections of the extract, initially at short intervals that are gradually extended, with increasing doses according to the protocol, over a period of weeks to months (initial treatment, or induction phase). This is followed by maintenance treatment, during which a constant amount of the extract is administered at longer intervals (typically 1 ml every 4 weeks). According to the guidelines of the International Committee on Allergic Diseases of Animals (ICADA), ASIT should be continued for at least 12 months before evaluating clinical success. If a horse responds favourably to ASIT, treatment should ideally be continued long-term, potentially for life.
-
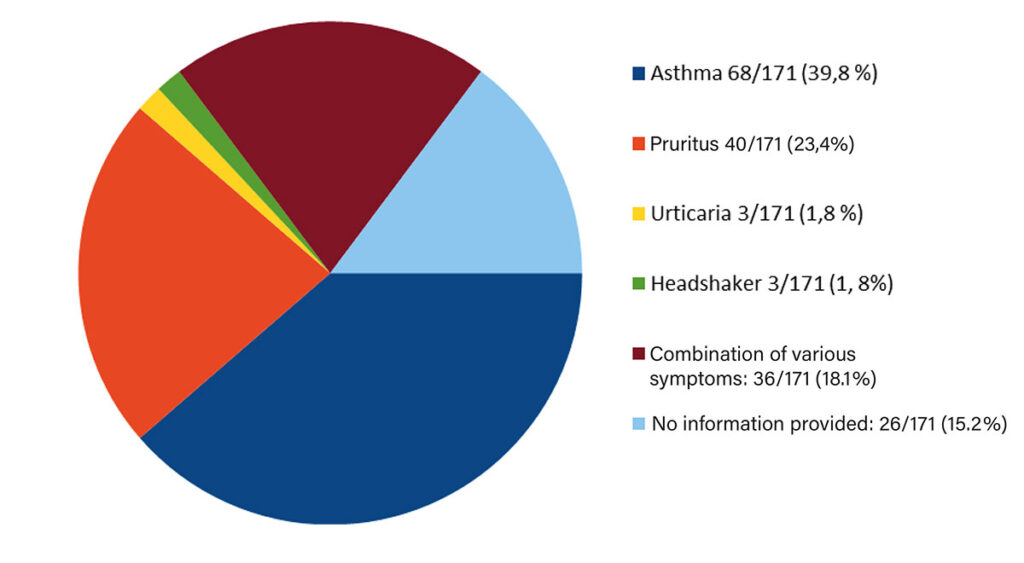
Fig. 1: Symptoms of the horses included in the study
Image source: LABOklin Laboratory for clinical diagnostics GmbH & Co. KG
-
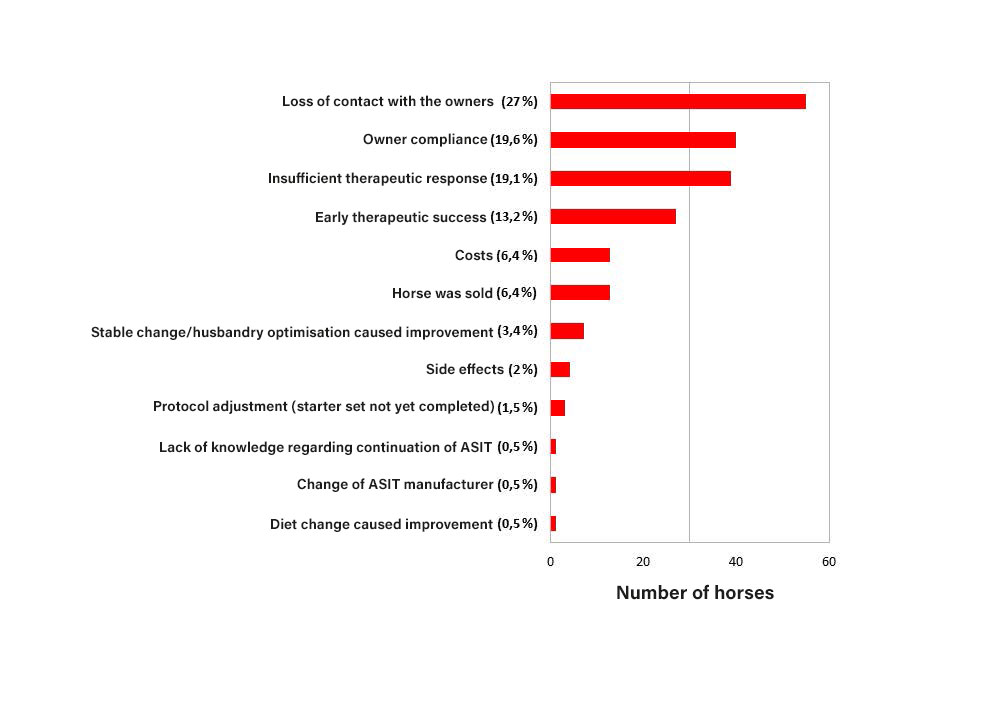
Fig. 2: Reasons for ASIT discontinuation
Image source: LABOklin Laboratory for clinical diagnostics Gmbh & Co. KG
Survey-based Study at Laboklin
The aim of the study was to identify the reasons for discontinuation of ASIT in horses during or after the induction phase (initial treatment, starter set).
The induction phase lasts approximately six months, which is considerably shorter than the recommended 12-month period for assessing the response to therapy.
Horses were selected from the ASIT treatment order lists of the Laboklin laboratory for the years 2021–2023, specifically those for whom no further ASIT treatments were ordered after the starter set. Of 4,271 initial treatments, 1,475 cases (34.5 %) did not receive any follow-up treatments.
To determine the reasons for discontinuation of ASIT, the treating veterinarians were contacted using written questionnaires. They could select one or more possible reasons. The collected data were analysed descriptively.
Reasons for ASIT Discontinuation
A total of 171 responses reporting 204 reasons for why no follow-up ASIT treatment was ordered after the initial treatment were analysed. Patients who did not receive further ASIT due to death (n = 15) were excluded from the analysis.
The horses included in the study exhibited the following symptoms: asthma (n = 68, 39.8 %), pruritus (n = 40, 23.4 %), urticaria (n = 3, 1.8 %), headshaking (n = 3, 1.8 %), or a combination of these symptoms (n = 31, 18.1 %). In 26 horses (15.2 %), the specific symptoms were not reported (Fig. 1).
The most common reasons for discontinuation of ASIT (Fig. 2) were loss of contact with the owner (n = 55, 27 %), lack of owner compliance (n = 40, 19.6 %), insufficient treatment response (n = 39, 19.1 %), or a good treatment response (n = 27, 13.2 %).
These four reasons accounted for over 80 % of treatment discontinuations. Other reasons included cost or sale of the horse (each n = 13, 6.4 %), adverse effects (n = 4, 2 %), and lack of knowledge regarding continuation of ASIT (n = 4, 0.5 %).
ASIT was also discontinued due to symptom improvement following a change of stable or optimisation of management (n = 7, 3.4 %) or a dietary change (n = 1, 0.5 %). In three horses (1.5 %), the induction phase had not yet been completed due to protocol adjustment, and in one horse (0.5 %), ASIT was continued but the ASIT manufacturer was changed.
In 116 questionnaires, it was reported that ASIT was administered according to the manufacturer’s protocol, while in four horses the protocol was individually adjusted. No additional symptomatic therapy was given in 93 horses; 11 horses received mucolytics alongside ASIT, 15 underwent inhalation therapy with glucocorticoids and bronchodilators, and six patients were treated with systemic glucocorticoids. In eight horses, it was reported that symptoms worsened again after ASIT was discontinued.
How Can ASIT Discontinuation Be Reduced and Treatment Success Optimised?
Induction treatments usually last for six months, which is considerably shorter than the recommended 12-month period for evaluating therapeutic success.
It can take up to a year for the full clinical benefit of ASIT to become apparent. In this Laboklin study, follow-up treatment was not ordered for over one third of initial treatments, resulting in premature discontinuation of ASIT after the induction phase.
Loss of Contact with Owners and Lack of Owner Compliance
The most common reasons for ASIT discontinuation were loss of contact between veterinarian and owner (27 %) and lack of owner compliance (19.6 %), together accounting for nearly 50 % of all discontinuations. In a Laboklin study on ASIT discontinuation in dogs, these were likewise the most frequently reported reasons. Owner compliance – the cooperation of the owner in implementing the recommended therapeutic measures – is a crucial factor for treatment success. Owners should be thoroughly informed about the treatment protocol, duration of therapy, the delayed onset of ASIT effects, and the expected costs, in order to align their expectations appropriately. Continuous communication is key to ensuring good owner compliance, particularly during the first year of therapy. Regular check-ups or telephone contact with owners not only maintain communication but also ensure continuous monitoring of the patient.
Treatment Success Lower than Expected
The third most common reason for ASIT discontinuation was insufficient therapeutic response (19.1 %). This was often reported in combination with poor owner compliance. Due to the delayed onset of ASIT effects, treatment success should be evaluated no earlier than one year after initiation.
Discontinuation of ASIT due to perceived lack of efficacy after the induction phase represents an issue of owner education, as these horses were prematurely classified as non-responders.
Veterinarians should clearly communicate the delayed onset of ASIT effects in order to manage owner expectations and prevent premature discontinuation during the first year of therapy.
In the current study, 93 horses reportedly did not receive any additional therapy, while only 15 horses were treated symptomatically during the induction phase of ASIT to alleviate allergic signs. Symptomatic treatments – such as glucocorticoids, antihistamines, bronchodilators, and mucolytics (in cases of equine asthma) – are often necessary during the first months of ASIT to rapidly reduce clinical symptoms until the effects of the immunotherapy take hold.
This approach is also an important factor in improving owner compliance. The duration and dosage of medications should be kept as low as possible. Symptoms should be reduced but not entirely suppressed, as complete suppression may obscure the need for protocol adjustment.
It is also important to correctly define treatment success. ASIT is considered successful if treated horses show an improvement of more than 50 % in clinical symptoms, or if the need for additional symptomatic medications can be reduced by more than 50 %. Before a horse is classified as a non-responder, it should be carefully evaluated whether there is truly no improvement. This requires precise documentation of the frequency of allergic episodes, as well as the duration and dosage of any additional symptomatic therapy. In this study, four respondents reported that ASIT was discontinued due to insufficient effect, but symptoms subsequently worsened after cessation. In these cases, it can be assumed that ASIT did provide clinical improvement that was not adequately documented, leading to the erroneous classification of these horses as non-responders.
The average success rate of ASIT in horses, according to a review by Herrmann et al. (2023), was 75 % for equine asthma, 88 % for urticaria, 59 % for pruritic dermatitis, and 36 % for sweet itch (ASIT using insect allergens only). Limited data are available regarding the efficacy of ASIT for allergy-in-duced headshaking; one study reported good to very good responses in five of the six horses included.
The reason why horses treated solely with insect allergens show lower success rates is not fully understood. One possible explanation is that, due to the simultaneous presence of different allergens, it is clinically difficult to distinguish between environmental pollen allergies and sweet itch (insect bite hypersensitivity). Many horses are polysensitised, and in these cases, additional allergens besides insects should be included in ASIT. Another theory is that insect allergens in ASIT may induce a weaker immune response compared with other allergens. This could be related to the fact that only whole-body extracts are currently available for ASIT, whereas treatments using pure insect salivary proteins might be more effective. This hypothesis is supported by studies using recombinant allergens in ASIT. A recent study by Graner et al. (2024) treated horses with ASIT consisting of recombinant Culicoides allergens. Clinical response was significantly higher than in the placebo control group, with almost 90 % of treated horses showing at least a 50 % improvement in symptoms in the second year of treatment.
The duration of the disease may also influence the success rate of ASIT. Hunsinger (2003) reported that horses treated with ASIT within two years of the onset of allergic symptoms responded significantly better to therapy. In horses with sweet itch, the success rate of ASIT was 75 % when treatment was initiated within the first two years after disease onset. This rate decreased considerably when the start of ASIT was delayed.
Another measure to optimise treatment success may be the individual adjustment of the protocol regarding injection volume and/or treatment intervals. In this study, protocol adjustment was reported in only four horses; all other patients were treated according to the manufacturer’s protocol.
Continuous patient monitoring is necessary to identify the need for protocol adjustment.
Discontinuation despite successful response
The fourth most common reason for stopping ASIT (13.2 %) was clinical improvement in the treated horses, leading to interruption of therapy. Most patients require long-term, often lifelong treatment to maintain control of allergic symptoms. Experience shows that symptoms typically recur after ASIT is discontinued. This was also observed in the present study in two patients whose ASIT was not continued due to marked improvement. Restarting ASIT can be demanding, often requiring repeated allergy testing, initiation of a new induction phase, and in some cases, a less favourable therapeutic response. Therefore, it is generally recommended not to interrupt ASIT in responders and to inform owners of the need for ongoing treatment. If clinical improvement is stable over several years during the maintenance phase, injection intervals may be gradually extended up to eight weeks.
Costs
In this study, costs were cited as the reason for ASIT discontinuation in 6.4 % of cases. For owners, the expenses associated with ASIT during the first year—including allergy testing and regular veterinary check-ups—may appear high.
However, in the long term, ASIT is considerably more cost-effective than purely symptomatic therapy, which can be very expensive in horses. Poorly controlled allergic horses often require more frequent veterinary visits, higher doses of symptomatic medications, and additional diagnostic or therapeutic interventions to manage potential side effects of these treatments. Providing this information can help motivate owners to continue ASIT throughout the first year of treatment and, during the maintenance phase, even in cases of moderate clinical response.
Side effects
In general, ASIT can be considered very safe in horses. In the present study, ASIT was discontinued due to adverse effects in four horses (2 %). In all cases, allergic symptoms worsened following the injections; additionally, one horse developed diarrhoea and another experienced circulatory problems. Worsening of allergic signs immediately after injections is one of the most common adverse effects, which was also observed in this study.
If this side effect occurs, the allergen extract dose should be reduced and the induction protocol individually adjusted. The importance of continuous communication between veterinarians and owners should be emphasised with regard to adverse effects. Owners should carefully observe their horses’ reactions to injections and immediately report any issues to the treating veterinarian to allow appropriate adjustments to the ASIT protocol.
The Laboklin team is available for consultation regarding protocol modifications.
Anaphylactic reactions (urticaria, angioedema, respiratory distress, circulatory collapse) are very rare. One horse in the current study experienced circulatory problems, leading to discontinuation of ASIT. The most common adverse effect during the induction phase is a self-limiting local reaction at the injection site; however, this was not cited as a reason for treatment discontinuation.
Conclusion
In summary, ASIT represents an important component of the multimodal management of allergic horses and is a lifelong therapy that requires close collaboration between owners and veterinarians. The first year of treatment necessitates intensive monitoring and constitutes the critical period for achieving therapeutic success. The most common reasons for discontinuation of ASIT are loss of contact with owners, poor owner compliance, and overly high expectations regarding rapid treatment effects. Improved education and communication, regular check-ups, and strict adherence to ASIT guidelines can increase the number of horses that respond successfully to ASIT and derive long-term benefit from the therapy.
Dr. Elisabeth Reinbacher
Our Services for Equine Allergies
- Screening tests
- Main tests for allergen differentiation (seasonal allergens, perennial allergens, insects, feathers/ hair/dander, feed)
- Screenings (allergy profiles – skin, allergy profile – respiratory)
- PAX complete (environmental allergens and/or food)
- Allergen-specific immunotherapy (ASIT)