The oak processionary (Thaumetopoea processionea) belongs to the order of moths (Lepidoptera), specifically a species of toothed moth. It is very similar to the pine processionary moth (Thaumetopoea pinivora). The adult moth flies between July and September.
The oak processionary occurs mainly in southern and Central Europe, but due to global warming, it is now also moving to northwestern Europe, wherever forests with a large number of oaks can be found. However, isolated trees along roadsides, in parks or urban areas can also be affected. In years of heavy infestation, hornbeams are particularly affected by these pests in addition to oaks.
Forest damage, in addition to the potential danger to humans and animals, should not be ignored. Although a single caterpillar feeding has no longterm negative consequences, repeated severe infestation and defoliation can make the oak more susceptible to secondary pests and lead to death.
The larvae (caterpillar) of the oak processionary are problematic, as they are covered with stinging hairs (setae) rich in taumetopoein that serve as a defence mechanism. Both larvae and adults (processionary moths) trigger a toxicirritant reaction (caterpillar dermatitis) called lepidopterism.
The female moth of the oak processionary lays 100-200 eggs on the branches of the crown of older oaks. A young caterpillar develops in the egg by autumn and overwinters in this form (larvae can survive temperatures to minus 30°C). The caterpillars are up to 5 cm long and can be recognised by their dark, broad dorsal line, velvety hair and long-haired warts. They live in groups of 20-30 and typically move in “nose-totail” processions (hence their name) when they are looking for food at night or when they want to pupate. This eponymous procession can be up to 10 meters long. In the caterpillar nests (webs), which are built on the trunk or the strong branches of the oak, moult occurs and serves as a refuge place. A nest can contain many hundreds or even thousands of larvae. From the third stage of the total of 5-6 developmental stages, the larvae develop stinging hairs with barbs (setae), which contain thaumetopoein, the nettle poison. The number and length of stinging hairs increase with each moult. During the feeding season in May and June they lose many hairs. If they feel threatened they release hairs into the environment as a defence.
All stages have natural enemies, from birds (such as the cuckoo or oriole), bats, ants, beetles, to fungi, bacteria, viruses, parasites/parasitoids. Note: a parasitoid is an organism that lives in close association with its host at the host‘s expense, eventually resulting in the death of the host (Wikipedia). An interesting detail is that the caterpillars cannot, for example, harm the cuckoo with their venom, as they are capable of regurgitating the urticarial hairs of the caterpillars. Thus, despite a large number of predators, their effectiveness in preventing infestations is limited.
The caterpillars feed on the leaves of the tree and eat the leaves, except for the veins. If this occurs over several years, it can cause severe damage to the tree. When a tree “defoliates“, the larvae leave the nest at night and crawl in a long procession to a new tree where they build a new nest. A single tree can host between 10,000 and 100,000 processionary caterpillars.
-
Fig. 1: Processionary procession
Picture Credits: Dr. Francesco Albanese
-
Fig. 2: A nest of processionaries in a pine
Picture Credits: Dr. Carmen Lorente
-
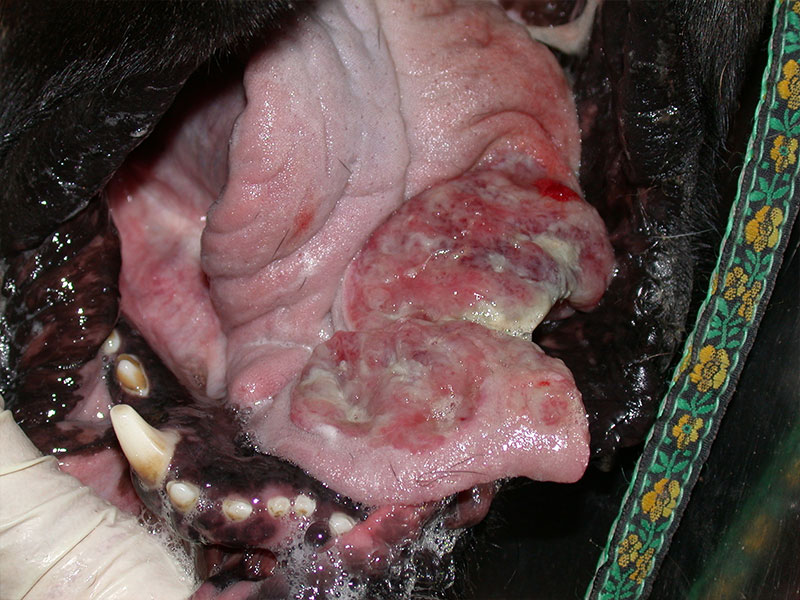
Fig. 3: Severe lesions to the tongue of a dog
Picture Credits: Dr. Francesco Albanese
-
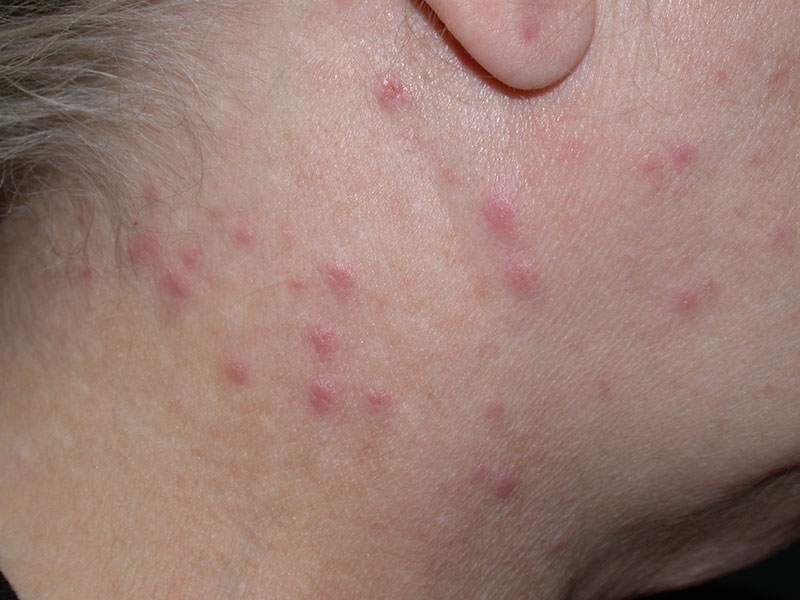
Fig. 4: Processionary dermatitis
Picture Credits: Dr. Francesco Albanese
The pest is combated particularly in the vicinity of human settlements. Measures such as treatment with insecticides, collection, flaming, chemical binders, suction or application of the bacterium Bacillus thuringiensis are carried out. Biological control is performed with insects parasitising eggs, beetles and microbiological control. A general distinction is made between organisational (blocking forests), mechanical (see above: collecting etc.) and chemical measures, which are only applicable during the first two larval stages.
The stinging hairs on the caterpillar are easily shed and are often carried long distances by the wind. The skin of the old larvae remains in the web, where the concentration of stinging hairs is exceptionally high. Caterpillar hairs collect on the ground in affected areas, where they can remain active for years.
During May and June, the hairs of the third larval stage are particularly dangerous for humans and animals. The hairs adhere to clothing and fur, they produce toxic reactions when in contact with the skin. The almost invisible stinging hairs can penetrate the skin and mucous membrane and get trapped there. Taumetopoein is a protein that leads to non-IgE-mediated degranulation of mast cells, causing an inflammatory effect in humans and animals. Contact with the caterpillar can cause hives, toxic dermatitis or itchy papules in people. Skin lesions occur, particularly in uncovered skin areas. They can also cause eye problems and respiratory signs if the hairs are inhaled. It is primarily a toxic-irritative, rarely an allergic reaction, but the IgE-mediated allergic hypersensitivity has also been described as leading to anaphylaxis. The most important measures are avoiding affected areas, protecting the skin with clothing, showering after possible contact and symptomatic therapy.
The main clinical signs in the dog are lesions in the mouth (glossitis, tongue oedema and necrosis, lingual and sublingual ulcers). In the study by Niza et al. (2008), ptyalism, dysphagia, and pain occurred in all 41 dogs described and lesions also occured in the eyes (conjunctivitis, keratitis, blepharitis, corneal ulceration).
The accumulation of antigen antibody complexes leads to the formation of microthrombi, which block the microcirculation, producing tissue necrosis. Caterpillar dermatitis should be included in the list of differential diagnoses when tongue necrosis is present. Submandibular oedema, facial itching and vomiting have also been reported. A group of 21 dogs also developed lymphadenopathy, tachypnea, tachycardia, hyperthermia and facial oedema. Rhinitis, laryngeal oedema or even lung symptoms can also occur when the hairs are aspirated. Systemic clinical signs in the form of an anaphylactic reaction are uncommon.
General management is symptomatic and supportive. It consists of eliminating stinging hairs and controlling clinical signs. Immediate treatment is aimed at removing the toxin and contact with the stinging hairs as soon as possible. Bath therapy or rinsing with saline or just water is recommended. The temperature of the solution is also of importance as heat inactivates the toxin. Washing should be carried out within the first two hours, after which time the risk of necrosis subsequently increases significantly. The administration of glucocorticoids or antihistamines is recommended, as well as analgesics in case of pain. If necroisis is present antibiotics would be indicated. In Niza et al. (2008), dogs that did not show necrosis took between 5 to 12 hours to heal, with tongue focal superficial necrosis 3 to 5 days, and if extensive necrosis, up to 15 days. All dogs with deep necrosis lost part of their tongue and required surgical debridement.
It is striking that most of the dogs in the study were less than one year of age, which could be related to the fact that young dogs tend to be more curious.
In human medicine, 2% lidocaine is used on the lesion.
Conclusion:
Caterpillar dermatitis due to the oak processionary should be considered in the differential diagnosis of dogs with non-specific clinical signs such as glossitis, tongue necrosis, salivation, eye inflammation, respiratory signs, urticaria, itching with a history of stay in an oak grove or area with the presence of oaks. Depending on the climatic region, there are peak times; however, since the stinging hairs persist for years, there is a general potential danger in the affected areas. The absolute immediate measure in the event of contact is “decontamination“ of the animal that should be carried out within the first two hours.
Dr. Regina Wagner
References:
-
DeBoer J.G. und Harvey J.A. (2020) Range-Expansion in Processionary Moths and Biological Control. Insects, 11, 267.
-
Kaszak et al. (2015) Pine processionary caterpillar, Thaumetopoea pityocampa Denis and Schiffermüller, 1775 contact as a health risk for dogs. Annals of Parasitology, 61 (3), 159-163.
-
Niza, M.E., et al. (2008) Effects of Pine Processionary Caterpillar Thaumetopoea pityocampa Contact in Dogs: 41 Cases (2002–2006). Zoonoses Public Health, 59, 35-38.
-
Sousa, C. et al (2009) Pine Processionary Poisoning: A 5 Years Retrospective Study, 19th ECVIM-CA Congress.