A wide range of different detection methods are available to veterinarians for the diagnosis of infectious diseases. However, the choice of test that is “just right” for the case at hand can be daunting.
Which is of higher clinical value, antibody- or direct pathogen detection? What is the concrete difference and at what stage of infection is each method best suited? This fact sheet is going to present the basics of each diagnostic tool and will discuss when each method is best used.
There are 3 main reasons why diagnostic tests for infectious diseases are carried out in veterinary practice:
- Verification of presence of infectious agents in acute and chronic stages of disease
- Detection of pathogen shedding in subclinical infections (to minimize transmission potential for other animals)
- Confirmation that an animal is free of infection (for example in breeding animals and for import / export)
Direct pathogen detection methods will identify the causative pathogens themselves (or at least parts of their genome or produced antigens).
Antibody detection is an indirect diagnostic method, which will uncover a previous contact by demonstrating an immune reaction against a specific pathogen. Different laboratory tests are available, using a variety of different methods and designs to detect either antibodies and / or infectious agents (Table 1).
-
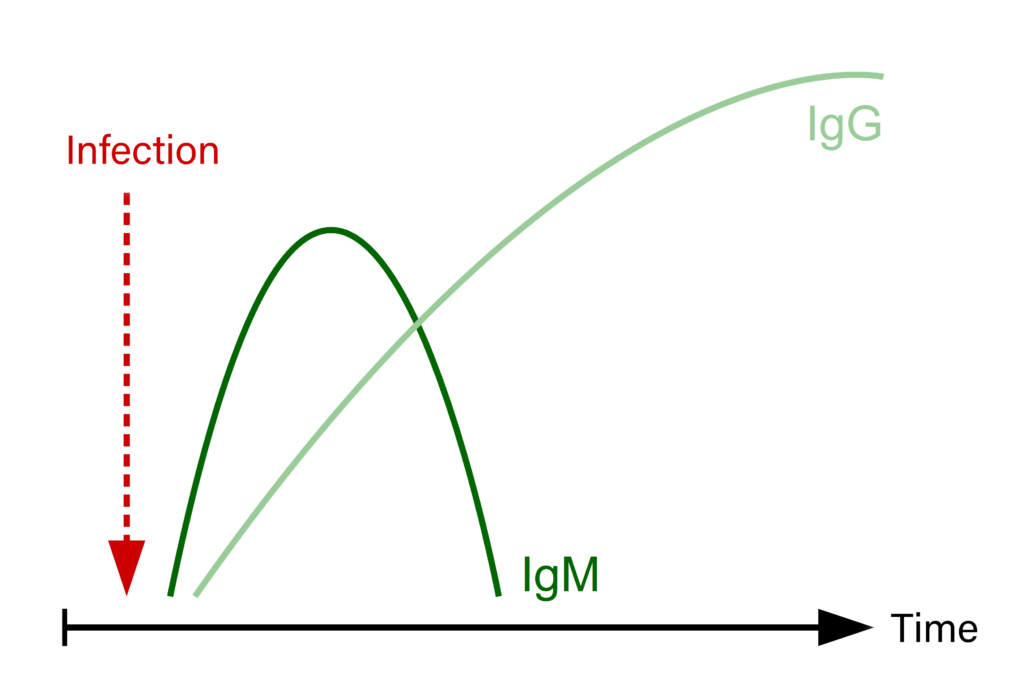
Fig. 1: Development of IgM and IgG titres following an infection
Source: Laboklin
-
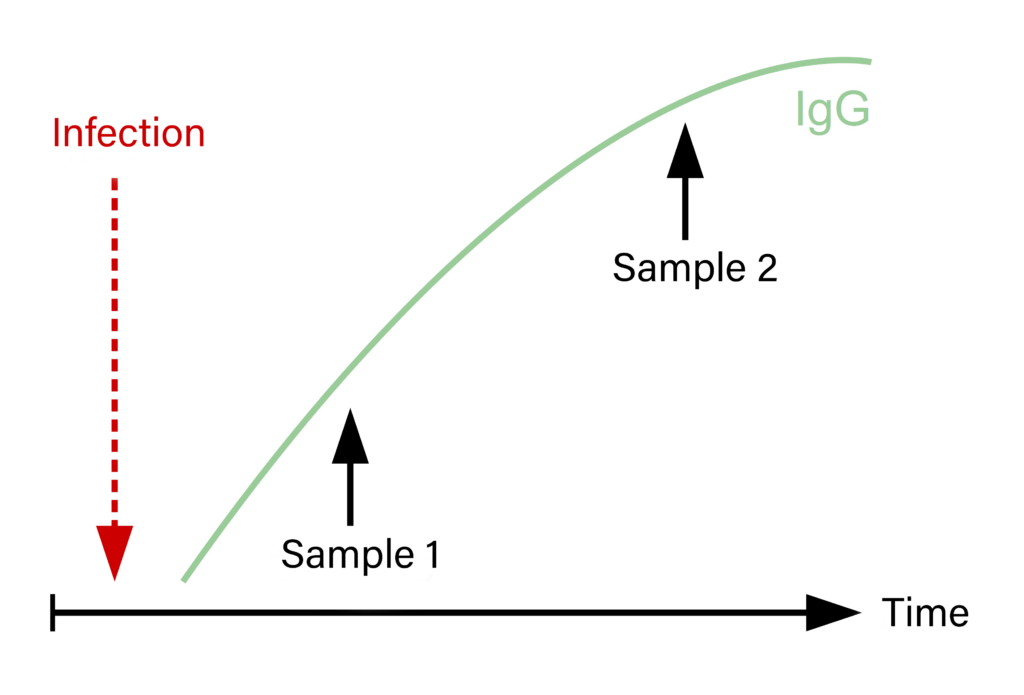
Fig. 2: Serum pairs and titre increase after a recent infection
Source: Laboklin
Table 1: Diagnostic methods for detection of infectious diseases and their use; Source: Laboklin
| Method | Direct pathogen detection | Detection of antibodies |
| Microscopy/Electron microscopy | X | |
| Microbiological culture | X | |
| Immunohistochemistry | X | |
| Polymerase-Chain-Reaction (PCR) | X | |
| Enzyme Linked Immunosorbent Assay (ELISA) | X | X |
| Immunfluorescence test (IFT) | X | X |
| Virus Neutralisation test (VNT) | X | |
| Western Blot (WB) | X | |
| Lateral Flow tests | X | X |
| And others |
Different test methods can lead to differing test results and there is a great variety and discrepancy in test sensitivity and specificity. It is important to keep in mind that no test will ever provide 100% sensitivity and specificity. In many cases, a combination of different diagnostic methods will be the most helpful and meaningful.
Direct pathogen detection
There is a variety of different test methods for the direct detection of pathogens. Examples are microscopy, snap-tests, Immune Fluorescence Tests (IFT) and Enzyme Immunoassays (EIA), which can detect pathogen antigens. In addition, there is also Polymerase Chain Reaction (PCR, detection of pathogen specific genome sequences) and microbiological culture. For all these diagnostic tools, there is a distinction between targeted and non-targeted methods.
The choice of sample material is of essential importance and dependent on where the pathogen is to be expected at the time of sampling. Depending on infectious agent and test method, different materials would be valid: blood, swabs, faeces, urine, aspirate, tissue, skin scrapings, hair and so on. Which of these materials can be used is subject to various factors. Knowledge of the pathogenesis of specific infectious diseases, especially target organs and route of shedding, are crucial. In addition, the current stage of infection at the time of sampling as well as age and immune (and vaccination) status of the patient are of importance.
Preanalytics
Before submitting a sample for testing, it is important to know whether or not the infectious agents have to be alive and able to replicate. Depending on this, prompt processing of the sample (without time delay) or special transport media might be necessary, which is especially important for microbiological cultures. It is important to follow the specific instructions for the respective sample by the commercial laboratory of choice, to receive the most meaningful test results possible. For example, for PCR tests, we recommend to submit dry swabs without transport media, EDTA whole blood, fluids in sterile, uncoated sample tubes as well as unfixed native tissue.
In the best-case scenario, sampling should be done as early in the infection as possible and always before any therapy attempts have started.
While any negative test result can never rule out an infection with absolute certainty, a positive test result usually confirms the presence of an infectious agent. However, a positive result does not always present a correlation of pathogen and clinical disease. Careful attention has to be given to the interpretation of results of particularly sensitive test methods. The ability to replicate and therefore the infectivity of certain infectious agents can only be validated in microbiological culture.
Antibody detection
Serum antibodies can be of different origins:
- Maternal antibodies: These can be present in foals up to 6-8 weeks (rarely up to 6 months). This has to be taken into account when interpreting positive results in this age group.
- Vaccination: In general, no direct differentiation between antibodies formed by vaccination and infection is possible (exception are so called marker vaccines).
- Infection: Antibody titres persist for a long time after infection.
It takes a certain amount of time for the immune system to develop specific antibodies after contact with a pathogen (vaccination or infection). The first sub-group of immune globulins to appear are IgM-antibodies, which can be detected after around 1-2 weeks, depending on the infectious agent and the immune status of the host. IgG-antibodies can be detected after around 3 weeks and in most cases persist over a long period of time (Figure 1). For infections with certain pathogens, these time periods can vary and some infectious agents, for example mycoplasms, will not initiate antibody formation.
Therefore, a positive antibody titre can only be expected after a certain period of time. In the case of acute and peracute stages of disease, no antibodies can be detected and especially for viral infections, only a retrospective diagnosis is possible.
For some pathogens (e.g. West Nile virus, TBE virus and Borrelia), a simultaneous detection of both IgM- and IgG-antibodies is possible. Since both are present at different stages of infection, a concurrent detection of both will assist in determining if a recent infection is present (IgM ↑) or if the infection has persisted for a longer time (IgG ↑).
However, for most pathogens, only tests determining IgG are available. A single test of an individual sample will not provide a meaningful result. For example, a positive result could either point to a previous infection or a vaccination. If the sample has been taken too early, false negative results are possible.
In contrast, a comparison of a serum pair, that was taken around 2-4 weeks apart, will assist with diagnosis and facilitate the interpretation of test results, although in some cases, this will only be possible retrospectively. A 4-fold increase of the antibody titre, or a comparative significant rise of a detection value, indicates a recent exposition (vaccination or acute infection; Figure 2).
In cases with unclear results, or if only a direct pathogen detection has to be performed, it would be advisable to take a serum sample at the onset of the disease. This serum sample can be frozen at -20°C and can be examined as an “acute” sample together with a “convalescence” sample taken at a later date, to complete the serum pair.
Different diagnostic laboratories use different test values and also vary in test procedures and technical equipment, so results will not be directly comparable between different laboratories.
Therefore, serum pair samples have to be analyzed using the same setup and methods at the same laboratory.
To detect antibodies in serum, the most commonly used methods are Enzyme-linked immunosorbent assays or immunofluorescence tests. In-house rapid tests are also available for veterinary practice, usually in the shape of lateral-flow-devices. For more specific enquiries, Western Blot is also available for some pathogens, which can be used to confirm other test results. The most specific serological diagnostic method is the virus neutralization test, which determines a cytopathic effect in cell cultures. It detects antibodies which neutralize viruses and therefore inhibit the infectivity of these viruses. Therefore, this test will also provide an indication for the presence of protective antibodies in vivo. In contrast, the detection of antibodies using ELISA is no reliable indication of the presence of protective antibodies. Depending on the pathogen, cellular immune response might be of equal or higher importance as the humoral immune response.
For serological tests, the most appropriate sample materials are serum and plasma. These should be processed as soon as possible after blood sampling, to avoid hemolysis which can interfere with the tests. In some cases, antibodies can also be detected in other liquids, like cerebrospinal fluid or aqueous humor. Since antibodies are relatively stable in the samples, processing at the laboratory is not time-critical. Therefore, serum samples can be stored for a longer period of time, either refrigerated or frozen at -20°C.
The presence of a positive antibody titre against a specific pathogen does not provide conclusive evidence that said pathogen is indeed the causative agent of a disease. For the interpretation of these results, the whole picture, including clinical symptoms and, if available, epidemiological data, has to be taken in account.
Serological tests may offer advantages compared to direct pathogen detection, particularly, if the pathogens in question are only present in the peripheral blood seasonally or periodically or if they are only present in tissue stages.
Take home message
Due to the large variety of infectious agents, it is not possible to offer a generalised recommendation of which diagnostic test to choose. Both direct pathogen and antibody detection are valid and are of clinical use. Depending on the problem, they can be used side-by-side and complement each other (Table 2). In many cases, it is useful and necessary to perform several different test methods.
Before submitting samples for testing at diagnostic laboratories, veterinarians have to collect a sufficient anamnesis, perform a clinical exam and formulate a preliminary diagnosis, while also determining the likely stage of infection. In the end, pathogenesis of a respective infectious agent as well as available test methods will decide which diagnostic test can be performed. For the most meaningful results, the following points are of importance:
- Time of sampling
- Type, quality and quantity of the sample
- Potentially required stabilisers or transport media
- Time and conditions of transport
Dr. Michaela Gentil
You can find our wide range of services relating to direct and indirect pathogen diagnostics in horses in the “Infectious diseases” section at: www.laboklin.com
Table 2: Short and to the point – a few examples of infectious agents and possible detection methods (bold= method of choice); Source: Laboklin
| Pathogen | Direct pathogen detection | Antibody detection |
| Equine herpesvirus 1 | PCR of EDTA blood (only during fever phase!), deep nasal swab, dead fetuses and placenta, cerebrospinal fluid, all depending on clinical symptoms | Disease peracute to acute, high seroprevalence in the horse population due to widespread distribution of the virus and vaccination; retrospective detection via serum pair is possible |
| Borrelia burgdorferi | Direct detection is difficult; PCR can be attempted from synovia, skin- or joint biopsies, if necessary | IgM- and IgG-antibodies as screening test, Western Blot for confirmation and useful to differentiate vaccination / infection antibodies |
| West Nile virus | PCR of EDTA-blood often low clinical value, since viremia has subsided by the time clinical symptoms appear; virus detection by PCR can be attempted in cerebrospinal fluid or tissue (post-mortem) | Concurrent detection of IgM- and IgG-antibodies by ELISA; cross-reactions with other flaviviruses (e.g. TBE, Usutu) are possible! In positive cases, differentiation is carried out using VNT |
| Dermatophilus congolensis | Detection in dandruff and scab material, using cytology (limited sensitivity) or PCR | No test available |
| Equine infectious anaemia virus (EIA) | High genetic variability of the virus, detection difficult | Once infected, animals remain virus carriers for life (persistent infection) and seropositive; diagnosis via Coggings-test or c-ELISA. However: Incubation period is up to 3 months, tests will have to be repeated during this time! Important: Foals of infected mares can be positive for up to 6 months due to maternal antibodies. |