Clinically, non-inflammatory alopecia is the absence of hair without other skin lesions.
There are various mechanisms of non-inflammatory alopecia:
- Follicular cycle arrest in endocrine diseases, telogen effluvium, anagen defluxion, post clipping alopecia. It usually produces partial or complete alopecia with symmetrical, generalised or regional distribution.
- Congenital or acquired genetic defects: congenital alopecia (fig.1), follicular dysplasias and dystrophies, alopecia X, pattern baldness (fig. 2). It appears with partial or complete alopecia of regional or generalised distribution.
- Inflammatory origin but clinically noninflammatory: alopecia areata, post-injection vasculitis, cicatricial alopecia, traction alopecia. Usually produces focal or multifocal complete alopecia.
Alopecia due to follicular cycle arrest
Hair is produced in the anagen phase. The telogen phase is a resting phase, in which the hair remains in the hair follicle until the follicle enters the anagen phase and synthesises a new hair that pushes out the «old» hair, producing the shedding of hair (old hair is replaced by new hair). If there is no activation of the hair follicle cycle, the old hair falls over time, producing alopecia.
Post-clipping alopecia: This occurs in plush coat breeds (e.g. Nordic) whose hair cycle has a very long catagen and telogen phase, and the anagen phase can take up to a year to activate. If shaving is carried out during the follicular inactivity phase, the hair can delay its emergence for months. Biopsy confirms the diagnosis by ruling out other processes.
Endocrine diseases
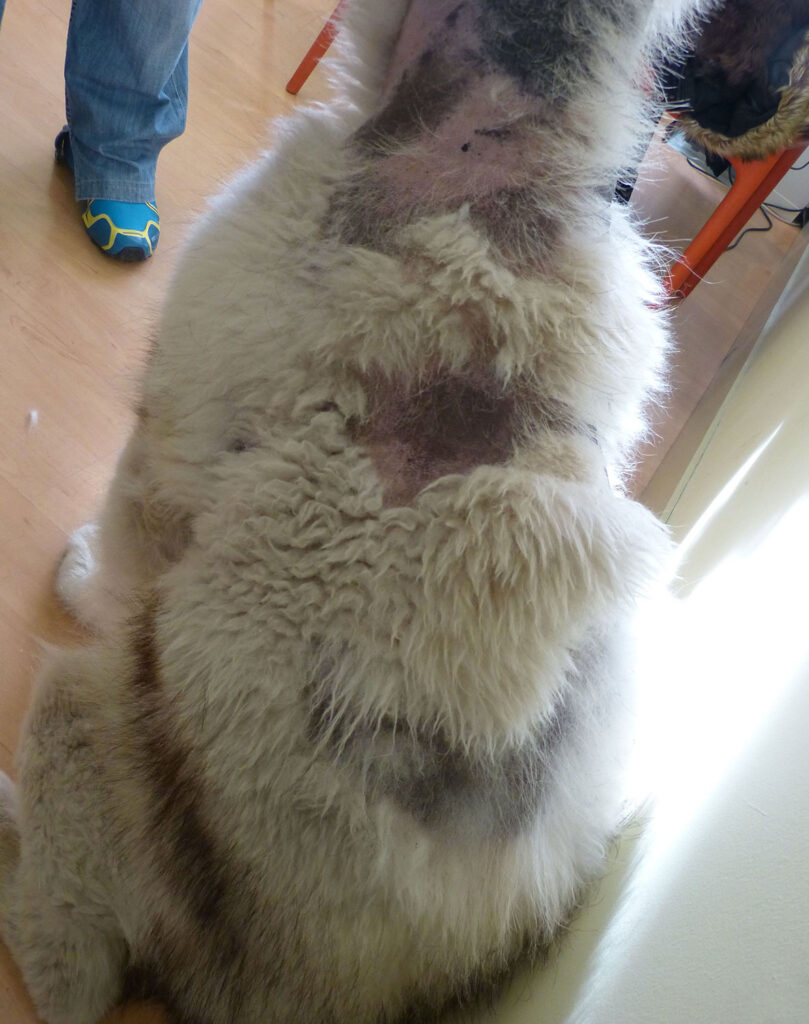
A defect or excess of certain hormones inhibits or prevents the activation of the anagen phase. The first dermatological signs are the presence of dry, dull, discoloured hair, which falls out easily, leading to progressive alopecia (fig.3). Alopecia begins in areas with increased friction, such as the neck, pressure areas, flanks or thighs, and progresses to other trunk areas. It does not usually affect the head or limbs. The skin is generally hyperpigmented, and comedones, seborrhoea and secondary infections are common.
The biopsy suggests endocrinopathy but cannot differentiate between the different endocrine diseases.
Dermatological lesions may be the first clinical signs of the disease, but other systems and organs are affected, and if the condition is not diagnosed can produce serious health issues.
Hypothyroidism is the most common endocrine disease in dogs. Thyroid hormones are necessary for the activation of the follicular cycle. In addition to the characteristic progressive alopecia of endocrine disorders, hair loss on the tail (rat tail) and alopecia of the nasal bridge have been described.
Accumulation of glycosaminoglycans in the dermisleads to myxedema as a cause of the tragic face expression. Frequent secondary nfections are associated with impaired skin barrier and immune system function.
In addition to dermatological signs, general clinical signs, cardiovascular, ocular, reproductive, gastrointestinal, muscular and peripheral neuropathy may occur. Common clinicopathological changes are normocytic and normochromic anaemia, neutropenia, thrombocytopenia, hypercholesterolaemia, and hypertriglyceridaemia.
The presence of low thyroid hormone levels (TT4) is very common in sick animals and can lead to diagnostic errors. Diagnosis should be based at least on the measurement of TT4 and TSH together. Complex cases may require complete panels such as the Thyroid profile Dog, including T4, fT4, T3, fT3, TSH, thyroglobulin Ab, T4 Ab*, T3 Ab* and/or the TRH stimulation test.
Hyperadrenocorticism (HAC)
Hyperadrenocorticism can be spontaneous (associated with pituitary micro, macroadenomas or adrenal gland neoplasia) or iatrogenic.
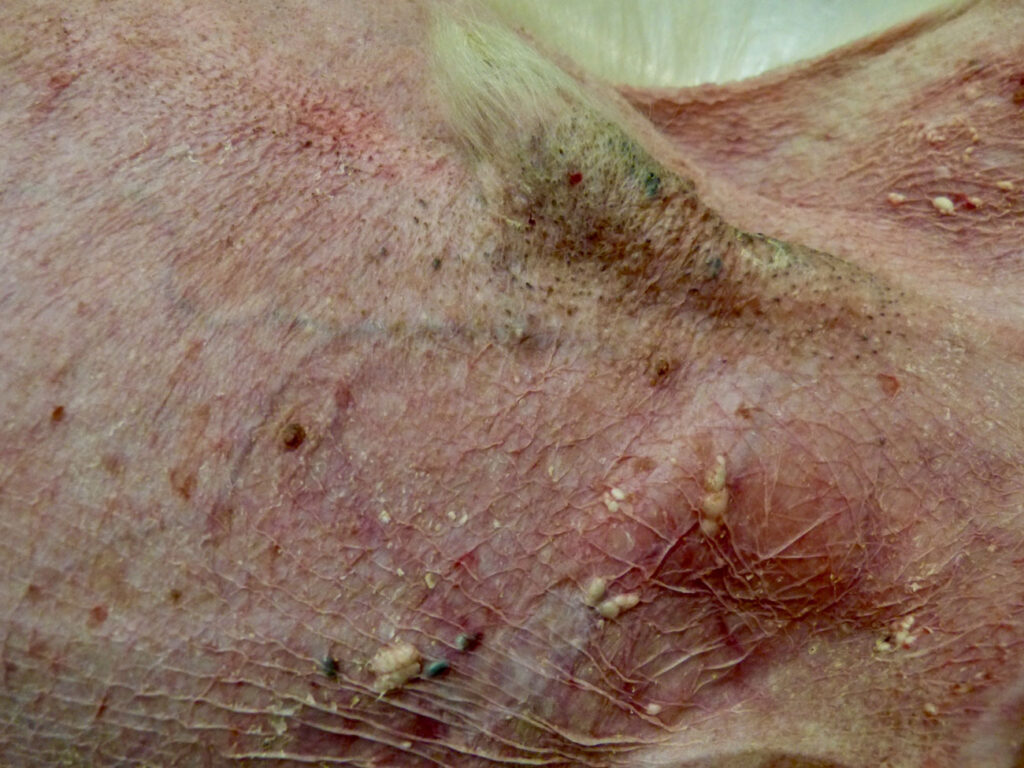
High levels of glucocorticoids inactivate the follicular cycle, cause atrophy of the adnexal, inhibit fibroblast proliferation and produce alterations in cornification. In 90% of cases, here are cutaneous manifestations: alopecia, skin atrophy (fig.4), comedones, poor healing, distension of scars, desquamation, xerosis and predisposition to secondary infections. Calcinosis cutis is not very common but is a very characteristic lesion of hyperadrenocorticism.
Other clinical signs include polydipsia/polyuria, abdominal dilatation, hepatomegaly, muscle atrophy, exercise intolerance, anestrus and metabolic complications: diabetes mellitus, systemic hypertension, urinary tract disorders, acute pancreatitis, CNS and neuromuscular disorders, and pulmonary thromboembolism.
The diagnosis of HAC usually requires the combination of several tests.
- Skin biopsy – is orientative
- Urinary cortisol/creatinine index is a screening test. It is not diagnostic, but average values in the absence of renal disease rule out HAC with high confidence
- ACTH stimulation test
- Suppression test with Dexamethasone at low doses (0.01mg/kg IV). Evaluate cortisol at T0 and four h and eight h after administration
- Suppression test with high dose Dexamethasone (0.1 -0.5 -1mg/kg IV).
- Ultrasonography of the adrenal glands.
- Computed tomography – in case of pituitary tumour
Sex hormone imbalance. The skin clinical picture is caused by oestrogen excess in males due to testicular tumours (fig.6), usually Sertoli cell tumours, but also seminomas and interstitial cell tumours. In females cystic ovaries can be a cause and less frequently ovarian neoplasia. It can be caused by exogenous oestrogen, including percutaneous penetration of oestrogen used by owners. The progressive bilateral alopecia usually starts in the perigenital area, extending to the abdomen, thighs, breasts, flanks and neck. Hyperpigmentation, lichenification, secondary infections and oily skin with a foul odour are common.
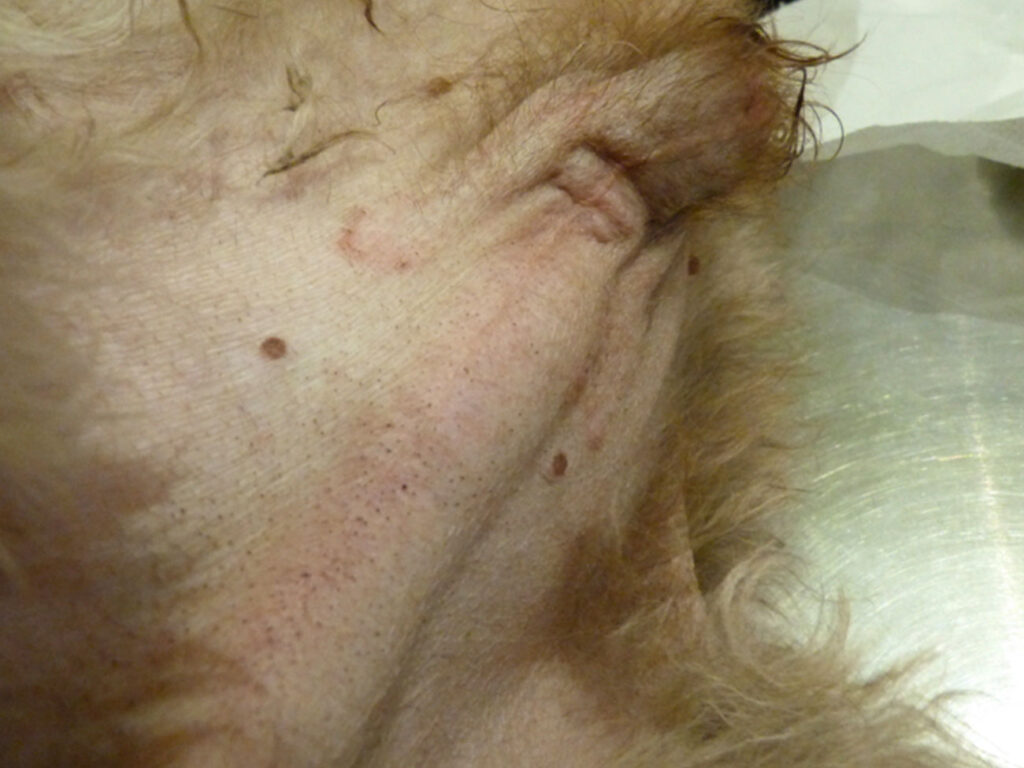
Other clinical signs include gynecomastia and enlarged nipples; enlargement of the vulva in females and in males: pendulous prepuce, linear preputial erythema (fig. 5) and attraction of the animal to other males.
Excess oestrogen can lead to irreversible bone marrow aplasia.
Diagnosis usually requires ultrasonography and biopsy. In addition, sex hormone (Adrenal Profile: 17 OH-progesterone, androstenedione, estradiol) can be determined.
Congenital or acquired genetic defects
Alopecia X– It is prevalent in Pomeranians and other breeds such as Chow-chow, Samoyeds, Siberian Huskies and miniature Poodles. There is a progressive loss of primary and later secondary hair until complete alopecia, starting on the neck, perineum or caudal thighs and progressing to complete alopecia of the trunk, excluding the head and limbs.
Diagnosis is based on a compatible biopsy together with tests that rule out endocrine disease. Alopecia X is an aesthetic process in an otherwise healthy animal, whereas an endocrinopathy is a disease that can have severe consequences for the animal‘s health.
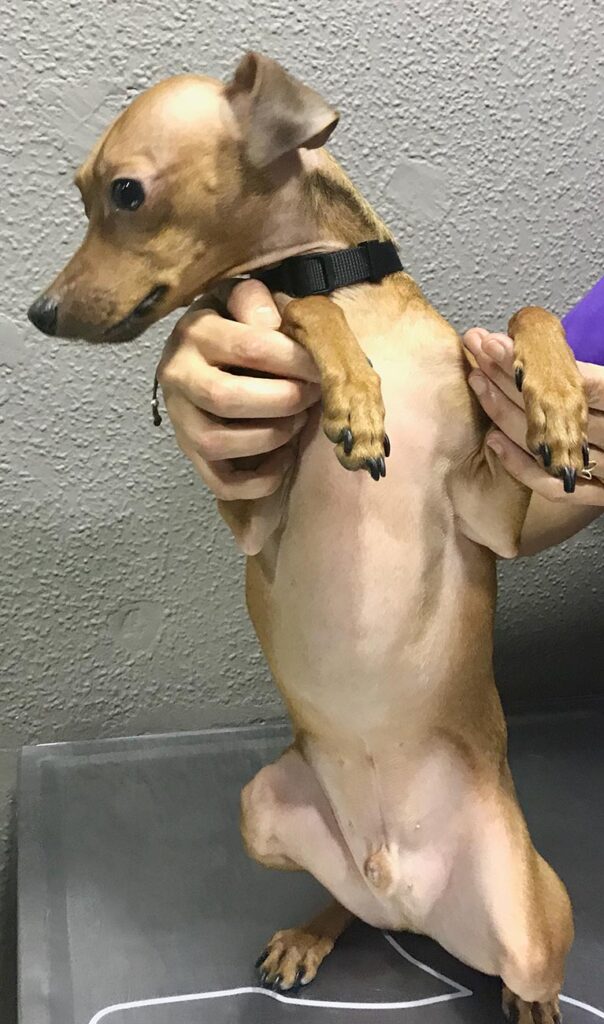
Congenital alopecia or hypotrichosis produces an absence of hair at birth or in the first months of life. There are selected breeds with congenital alopecia – e.g. Chinese Crested dog (fig.1).
-
Fig. 1: Congenital alopecia in a hairless dog.
Source: Dr Carmen Lorente
- Fig. 2: Pattern baldness with ventral, auricular and preauricular alopecia in a short smooth coat dog. Source: Dr Carmen Lorente
- Fig. 3: Alopecia and dry, dull, woolly coat in an Alaskan Malamute with testicular tumours (seminomas). Source: Dr Carmen Lorente
-
Fig. 4: Alopecia and skin atrophy in a dog with HAC. Dry, cigarette paper-thin skin and visualisation of blood vessels may be observed.
Source: Dr Carmen Lorente
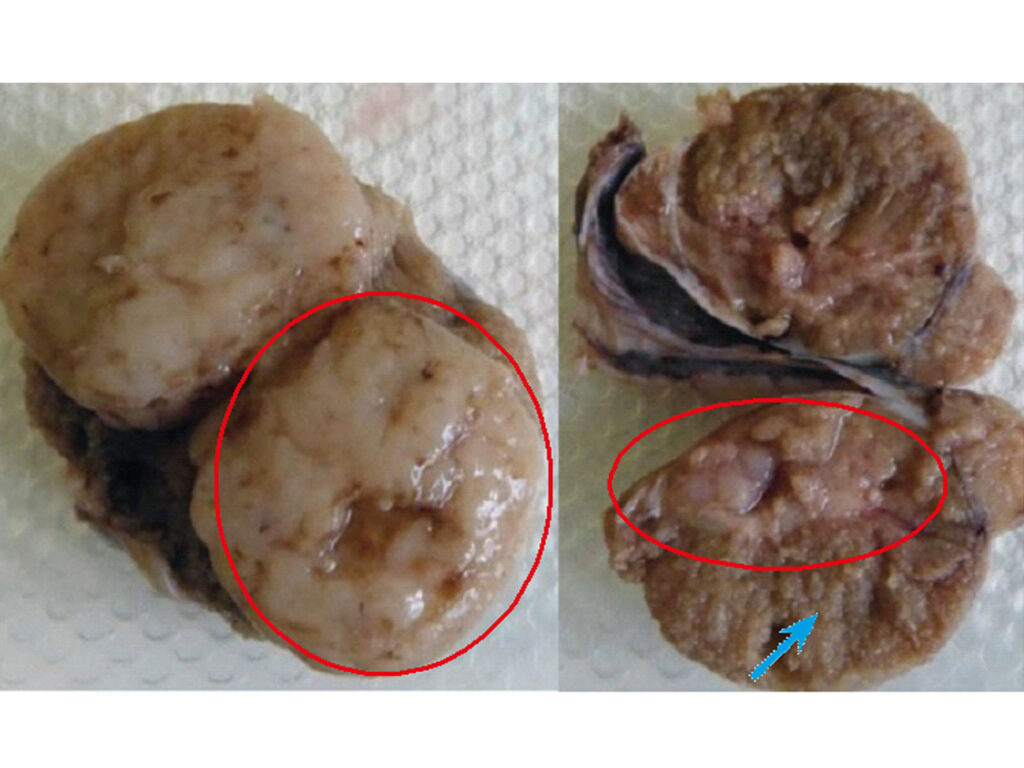
- Fig. 5 and 6: Linear preputial erythema in a dog with bilateral testicular tumours. The red circles enclose neoplastic tissue, and the blue arrow points to normal tissue. A biopsy is necessary to identify the type of neoplasm. Source: Dr Carmen Lorente
- Fig. 5 and 6: Linear preputial erythema in a dog with bilateral testicular tumours. The red circles enclose neoplastic tissue, and the blue arrow points to normal tissue. A biopsy is necessary to identify the type of neoplasm. Source: Dr Carmen Lorente
-
Fig. 7: Follicular dysplasia in a Spanish water dog. There are breed-specific follicular dysplasias with a characteristic distribution pattern of alopecia.
Source: Dr Carmen Lorente
Pattern baldness.
Predisposed breeds have thin hair standards (Dachshund, chihuahua, whippet, greyhound). Alopecia due to miniaturisation of the hair. It usually starts before the age of one year and progresses to alopecia in a specific area (ear pinnae, ventral area of neck, chest and trunk, caudal area of the thighs) depending on the breed affected (fig.2).
A biopsy confirms the diagnosis.
Cyclic, recurrent or seasonal flank alopecia.
Predisposed breeds are English and French Bulldogs, Miniature Schnauzer, Airdale Terrier, Doberman. There is an alteration of coat quality and hair loss (mainly localised on the flanks), which usually starts in late autumn or early spring, resolves partially or entirely in a few months and recurs the following season. Over time the alopecia is generally permanent. Diagnosis may require biopsy and ruling out possible endocrine diseases.
Follicular dysplasias (fig.7)
Follicular dysplasia associated with hair colour. It is the consequence of poor melanin transfer with the formation of large melanin aggregates that deform the hair shaft and eventually break it. The alopecia is initially partial and diffuse, giving a moth-eaten coat appearance and then may become complete with time. Depending on the colour of the coat, it is called:
- Dilute colour alopecia– affects animals with dilute colour (grey-blue, Isabella or dilute brown).
- Black hair follicular dysplasia (BHFD) – affects exclusively black hair, with evident alopecia circumscribed to black areas.
Trichography shows the presence of large melanosomes that distort and fracture the hair shaft.
The biopsy is diagnostic.
Clinically non-inflammatory alopecia due to an inflammatory process
Alopecia areata. Follicular bulbs are attacked by lymphocytes, leading to their destruction and consequent alopecia. Clinically a focal, regional, multifocal or rarely general non-inflammatory alopecia is observed. The hair may or may not grow back, and if it does, it usually grows white. Diagnosis requires a biopsy.
Vaccination or post-injection panniculitis.
Circumscribed alopecia associated with the inoculation site of a vaccine (mainly rabies) or an injectable. Non-inflammatory alopecia may appear or be accompanied by another lesion (nodulation, ulceration, oily discharge). Diagnosis requires a biopsy.
Scarring alopecia. Associated with trauma or recovery from a severe and deep inflammatory process. History and dermatological examination can diagnose the process. A biopsy is a complementary test for diagnosis.
Traction alopecia. Circumscribed alopecia, associated with ischaemia due to rubber bands or fastening ornaments. It usually results in a permanent aesthetic defect. History and dermatological examination can diagnose the process. A biopsy confirms the diagnosis.
Dr Carmen Lorente, DVM, PhD, DipECVD