Can a single blood sample simplify the invasive and expensive work-up of tumour patients? This is a question frequently faced by both owners and veterinarians. The general answer is: to some extent. Blood samples can indeed provide crucial information for diagnosis and monitoring.
This article explores the current status of this approach.
First of all, serological tests should be distinguished from liquid biopsy methods. Serological tests detect proteins produced by tumour cells in the blood, whereas liquid biopsy methods are employed to identify DNA, RNA, and nucleic acid-associated proteins in body fluids.
Haematology
Non-regenerative anaemia is often associated with tumour diseases such as anaemia of chronic disease. It can indicate bone marrow infiltration by neoplastic cells (e.g., in leukaemia, lymphoma, multiple myeloma, or other metastases) or oestrogen-producing tumours (such as Sertoli cell tumours). Different cell lines are typically affected in cases involving bone marrow, making it important to consider relevant tumours, especially when accompanied by neutropenia and/or thrombocytopenia.
Thrombocytopenia is commonly observed in spleen tumours, while thrombocytosis can also arise in various neoplastic conditions.
Massive lymphocytosis can occur with lymphoproliferative neoplasms.
Eosinophilia may arise, for instance, as a response to a mast cell tumour or in association with T-cell lymphoma.
Blood Chemistry
Specific blood chemistry parameters can offer valuable insights for an oncological work-up, including globulins, lactate dehydrogenase (LDH), and calcium (Ca).
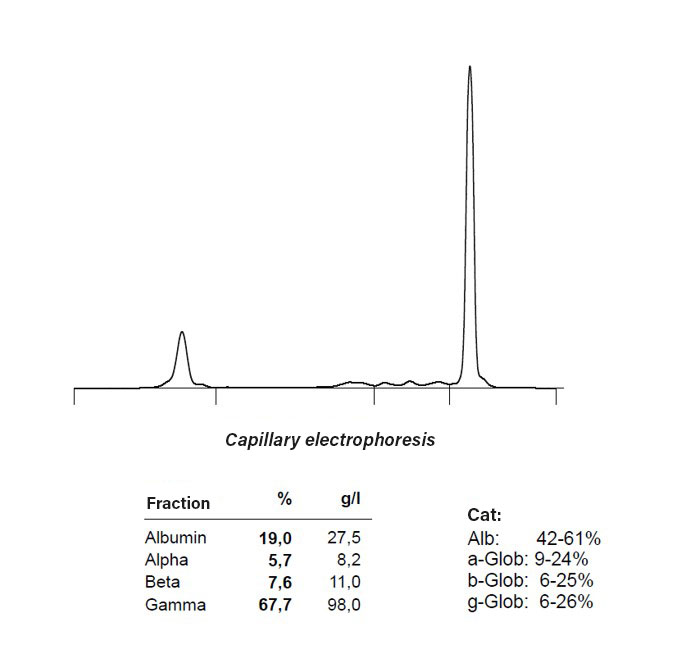
Hyperglobulinaemia is observed in antibody-producing tumours such as multiple myeloma, plasmacytoma, and B-cell lymphoma/leukaemia. Serum electrophoresis can provide evidence of this condition (see Fig. 2).
The elevated metabolism in tumour tissue and the associated rapid cell proliferation increase lactate dehydrogenase (LDH). High LDH levels in the blood of dogs may indicate malignant tumour diseases.
More importantly, an increase in LDH levels in lymphoma patients undergoing therapy can indicate a potential recurrence.
-
Fig. 1: The risk of tumours particularly increases with age. In addition to the clinical assessment, blood samples can offer valuable insights into the disease.
Image source: envatoelements
-
Fig. 2: Monoclonal gammopathy with hypoalbuminaemia in multiple myeloma
Image source: Laboklin
-
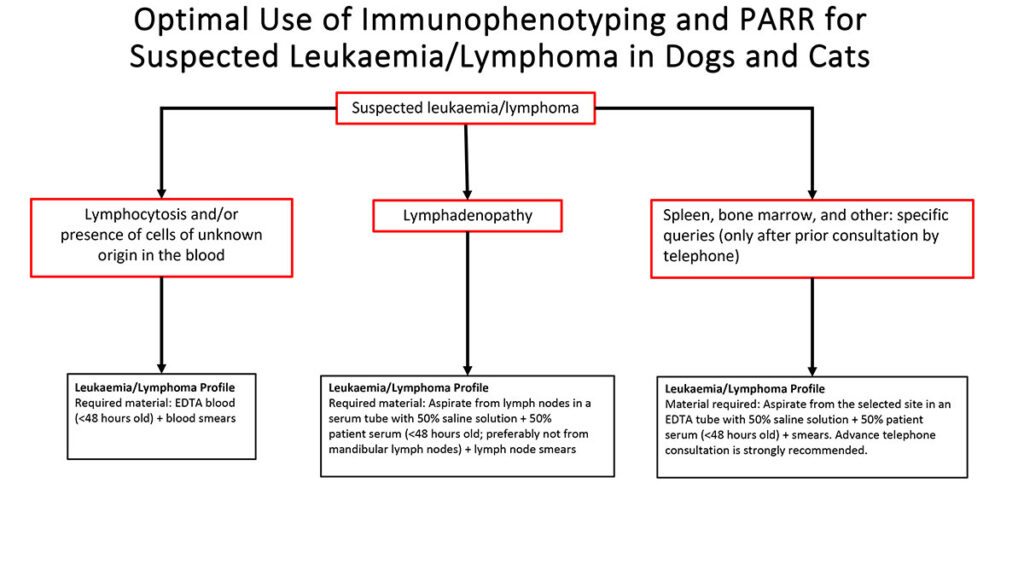
Fig. 3: Schematic diagram of the diagnostic procedure for suspected lymphoma or leukaemia
Image source: Laboklin
Various types of tumours can induce tumour-associated hypercalcaemia. The most common tumour types linked with hypercalcaemia include lymphoma, multiple myeloma, thymoma, and anal sac carcinoma. Parathyroid hormone-related protein (PTHrP) may contribute to the development of tumour-associated hypercalcaemia; however, other mechanisms such as parathyroid hormone, elevated levels of vitamin D metabolites, or the activity of specific cytokines can also be involved. Therefore, while many tumour diseases are associated with hypercalcaemia, not all show an increased PTHrP. Additionally, mildly elevated serum calcium levels do not necessarily confirm the presence of a tumour. In most cases, primary hyperparathyroidism is attributed to an adenoma of the parathyroid gland.
Additionally, elevated concentrations of acute phase proteins ‒ such as C-reactive protein (CRP) in dogs or serum amyloid A (SAA) in cats and horses ‒ can operate as indicators. These proteins are commonly elevated in cases of disseminated tumours (e.g., lymphoma or metastatic tumours) and the presence of extensive tumour necrosis.
Suspected Lymphoma or Leukaemia ‒ What to Do?
We have summarised the schematic work-up of lymphoproliferative neoplasms using immunophenotyping and clonality analysis of fluids in the overview provided (Fig. 3). For more specific details on this topic, refer to the Laboklin Aktuell from 05/2017.
Tumour Markers
Tumour markers are biochemical substances ‒ such as glycoproteins, hormones, enzymes, metabolic products, or DNA components ‒ present in increased quantities in the blood during tumour diseases. These markers may be produced directly by the tumour or may be stimulated by the tumour in endogenous cells. While they can be specific to a particular tumour type, this is not always true. It is important to be aware that benign conditions can also lead to elevated levels of classic tumour markers. Positive findings must always be distinguished from inflammatory diseases. A single abnormal value is not sufficient to confirm neoplasia. The primary use of tumour markers is in monitoring disease progression and assessing the risk of recurrence. It is advisable to establish a baseline value before starting treatment.
Thymidine Kinase (TK-1)
TK-1 is an enzyme involved in the incorporation of the amino acid thymidine into DNA.
Its concentration in the blood reflects cellular proliferation activity. Reference values are available for dogs, cats, guinea pigs, and horses. TK-1 levels are notably elevated in haematopoietic tumours and high concentrations are associated with shorter survival times in dogs with lymphoma. TK-1 is also helpful for monitoring therapy and early detection of recurrences in lymphoproliferative diseases. Some canine lymphoma show increased values a few weeks before a clinically evident relapse.
Alpha-1-Fetoprotein (AFP)
AFP is a glycoprotein produced in the yolk sac, liver, and gastrointestinal tract of the embryo, functioning as a transport protein similar to albumin. Post partum its production is limited to small amounts in the liver and intestines. Elevated AFP levels are observed in dogs with lymphomas and mast cell tumours. In humans, AFP is used to diagnose hepatocellular carcinoma and predict its prognosis. As the AFP concentration in the serum of dogs with hepatocellular carcinoma is higher than other liver diseases, it could be a valuable tool for the diagnosing and following up with hepatocellular carcinoma in dogs. However, AFP can also be elevated in some benign liver conditions, such as hepatocellular adenoma, inflammatory diseases, or chronic hepatopathy, and research is limited. Thus, AFP should be considered a potentially helpful component of an overall diagnostic approach.
Carcino-Embryonic Antigen (CEA)
CEA is a glycoprotein found in glandular tissues. Elevated serum concentrations are associated with inflammatory or malignant changes in these tissues. In human medicine, CEA is a valuable marker for lung, colon, breast, ovaries, and prostate cancers. It provides prognostic information, particularly for intestinal tumours. Studies in dogs have focused on cancers of the mammary glands, stomach, pancreas, and bronchi. Currently, its use in dogs is mainly limited to supplementary diagnostics and monitoring for recurrences or metastases.
Nucleosomes
A nucleosome consists of a DNA segment wrapped around histone proteins. These histones are increasingly released when cells die and can be detected in the blood. The commercially available Nu.Q®Test, initially developed for human medicine, has been evaluated for use in dogs. It has shown good sensitivity for detecting disseminated and aggressive tumours such as lymphomas, histiocytic sarcomas, and haemangiosarcomas. However, some solid, localised tumours (such as soft tissue sarcomas) are less frequently detected. It is important to note that free circulating nucleosomes and histones can also be elevated in dogs with inflammatory diseases and after trauma. For example, the test does not differentiate between neoplastic and inflammatory or infectious causes in a dog with a febrile illness. The test is intended primarily as a screening tool for clinically healthy dogs.
Genetic Tumour Risk and Mutations
Testing for germline mutations, often called genetic testing, identifies hereditary genetic defects that increase the risk of developing certain tumour diseases. This approach enables the assessment of tumour risk for individual dogs during screening examinations. Laboklin currently offers genetic tests for renal cell carcinoma with associated dermatofibrosis in German Shepherds, familial thyroid carcinoma in German Longhairs, and squamous cell carcinoma of the toe in Black Poodles and Black Giant Schnauzers. Additionally, there is a test for histiocytic sarcoma in Bernese Mountain Dogs, although Laboklin does not provide this test.
In addition to hereditary mutations, alterations can also occur in somatic cells. The BRAF protein, which is involved in normal cell growth, is regulated by various cell signals. A change in the BRAF gene can lead to overactivation and uncontrolled growth of the affected tissue. In dogs, identifying a BRAF alteration helps differentiate between bladder, urethra, and prostate carcinomas and benign proliferations with high specificity. The detection of the presence of a BRAF mutation needs cellular material. The test can be performed on cell aspirates (obtained using catheter suction technology) or cells in urinary sediment, making it a less invasive option for evaluating findings suspicious for tumours. While a positive result is highly specific, a negative result does not exclude the presence of a tumour. False-negative results may occur due to insufficient cellularity of relevant cells, or a tumour without the specific mutation.
Additionally, copy number alterations (CNA) can be assessed and significantly altered in urothelial carcinomas compared to normal, benign, or inflammatory bladder conditions.
Conclusion
In summary, tumour diagnostics can be like a mosaic, with various elements coming together to form a complete picture. Depending on the case, additional examinations ‒ such as imaging procedures, cytological analyses, or histological evaluations ‒ may be required.
Sophie Burde, Dr. Katrin Törner,
PD Dr. Heike Aupperle-Lellbach
Our services relating the subject
- Tumour diagnostics small / large
- Thymidine kinase
- CEA
- AFP
- BRAF and BRAF comp.
- Lymphocyte clonality (PARR)
- Leukaemia immunophenotyping
- Leukaemia/lymphoma profile