Dogs with otitis externa (O. e.) are frequently presented to the small animal practice. Clinical signs are head shaking, scratching, restlessness and an unpleasant odour. In most patients with O. e., it is a multifactorial disease. For one, there may be predisposing factors (breed, “swimmer’s ear”, incorrect ear cleaning). Apart from that, it can be distinguished between primary causes (especially foreign bodies, parasites, allergies) and secondary causes which maintain the disease (untreated/ incorrectly treated inflammation, pathological changes). A small amount of bacteria belonging to the physiological skin microbiome (coagulase-negative staphylococci, alpha- and non-haemolytic streptococci, Bacillus spp., Corynebacterium spp.) can be detected in the healthy external auditory canal. Furthermore, a small number of bacteria classified as pathogenic (S. pseudintermedius, beta-haemolytic streptococci, E. coli, Proteus spp., pseudomonads) can be found there. A low level of yeasts (M. pachydermatis) is also considered normal. As a result of the changed conditions in the affected ear, especially in chronic inflammation, both bacteria and yeasts can multiply well and it is sensible to take swabs from the affected ear(s) and let them be examined microbiologically. At the beginning, a cytological specimen should be prepared. On the one hand, cytology shows the amount of bacteria, and on the other hand, it is possible to differentiate between cocci and bacilli as well as to detect Malassezia and inflammatory cells. If suspicious structures are detected in cytology, it is always advisable to grow a culture and subsequently perform an antibiogram. If antibiotic and/or antimycotic treatment is necessary, various commercial ear preparations with different active ingredients are available.
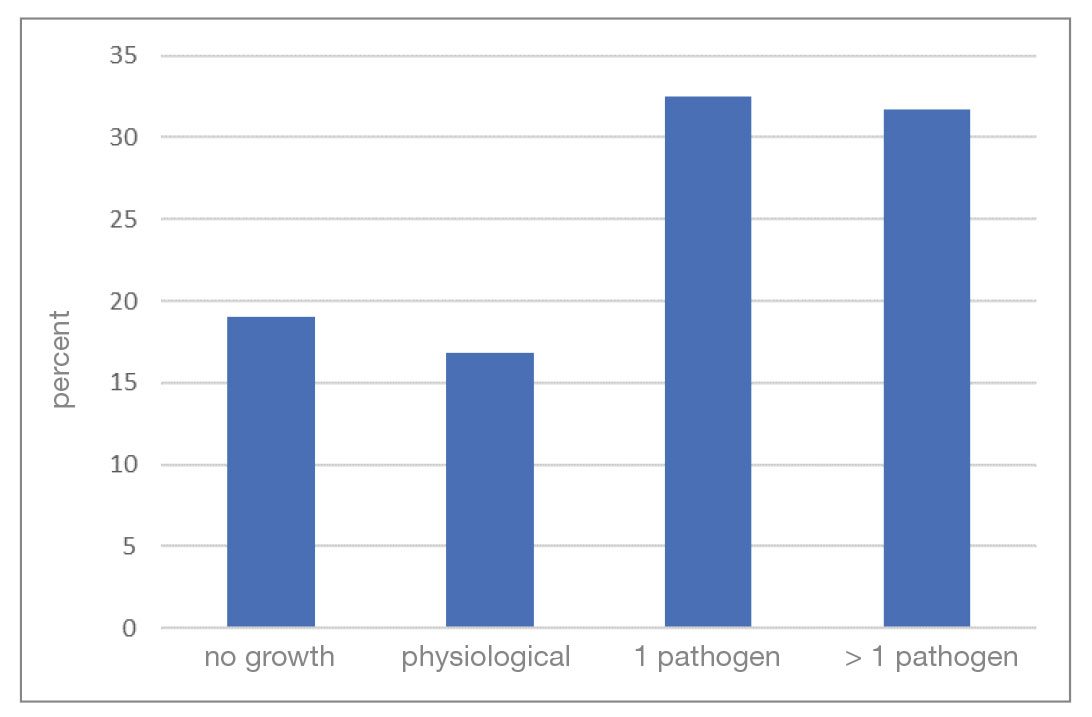
Pathogen spectrum: The analysis includes 8896 ear swab samples from dogs which were examined by culture in 2016 as part of routine diagnostics. 19% of the samples did not show any bacterial growth (culture negative). Bacteria could be detected in 81% of the samples (culture positive). Of these, a pure culture could be grown in 32.5% of cases, a mixed culture in 31.7% and only a physiological microbiome in 16.8% of cases (Fig. 1).
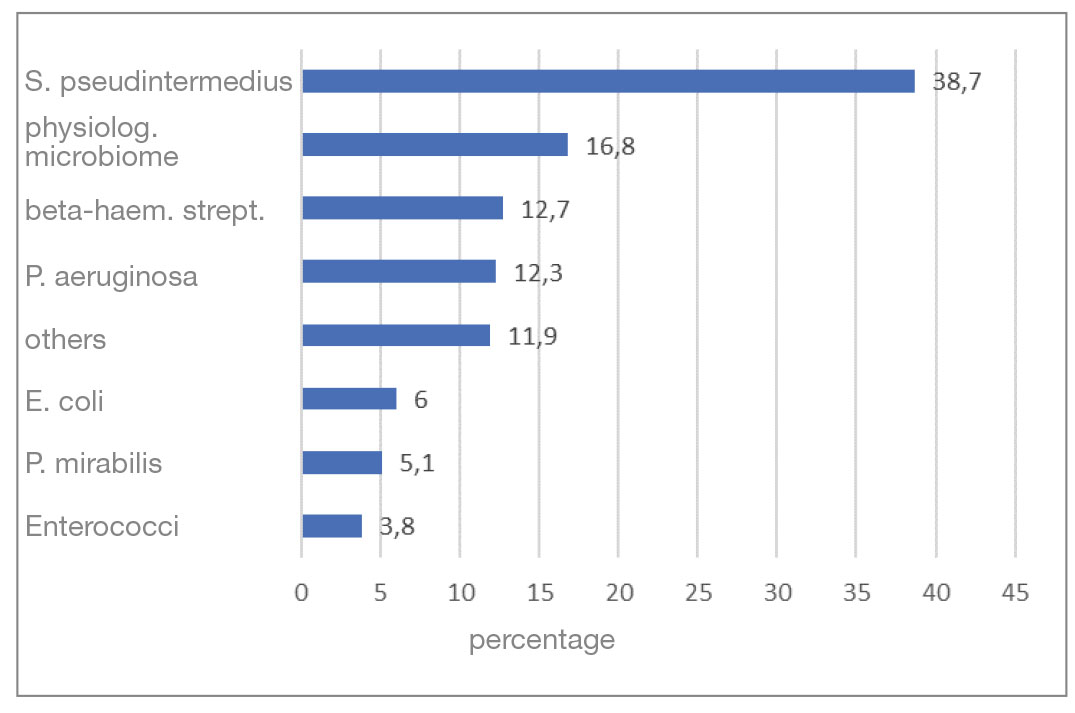
The most common bacterium detected in the bacteriologically positive samples was S. pseudintermedius (38.7%). Almost half of it could be detected in pure culture. Beta-haemolytic streptococci were cultivated in 12.7%, P. aeruginosa in 12.3% and “other” pathogens in 11.9% of the samples. This was followed by E. coli with 6%, P. mirabilis with 5.1% and enterococci with 3.8% (Fig. 2).
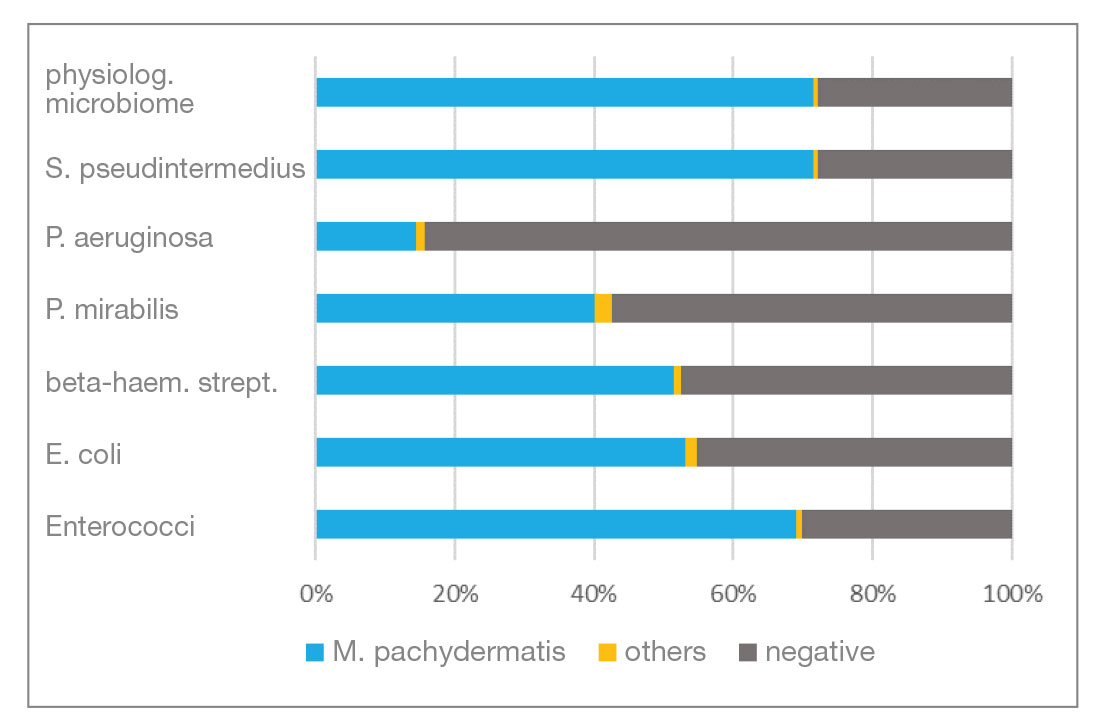
Mycological examination: Of the total number of samples, 78% were examined bacteriologically and mycologically and 22% by bacteriology only. 62.8% of the mycological samples were culture positive and 37.2 % were culture negative. M.pachydermatis accounted for the largest proportion of mycologically positive samples at 98.6%. Figure 3 shows the detection rate of Malassezia in combination with each type of bacteria detected as well as the number of negative results. Candida spp. and moulds (“others”) were only detected in individual cases.
Level of resistance: the resistance of the most frequently detected bacteria to the active substances contained in ear preparations available at the time of evaluation (Fig. 4 – 9) were examined.
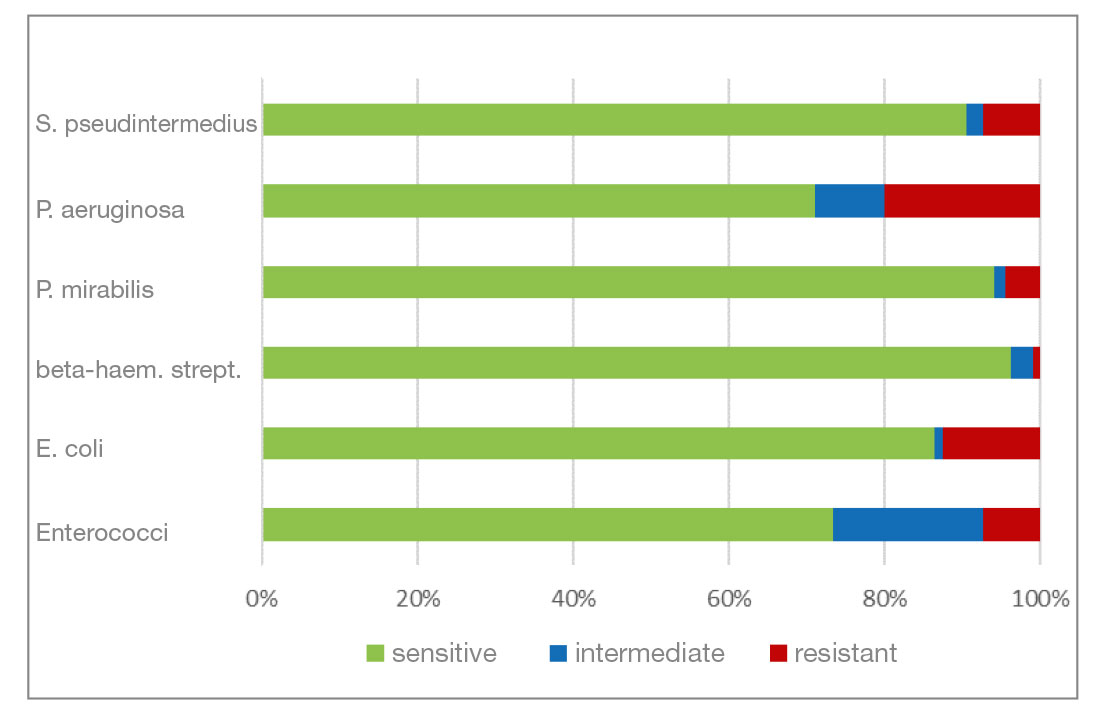
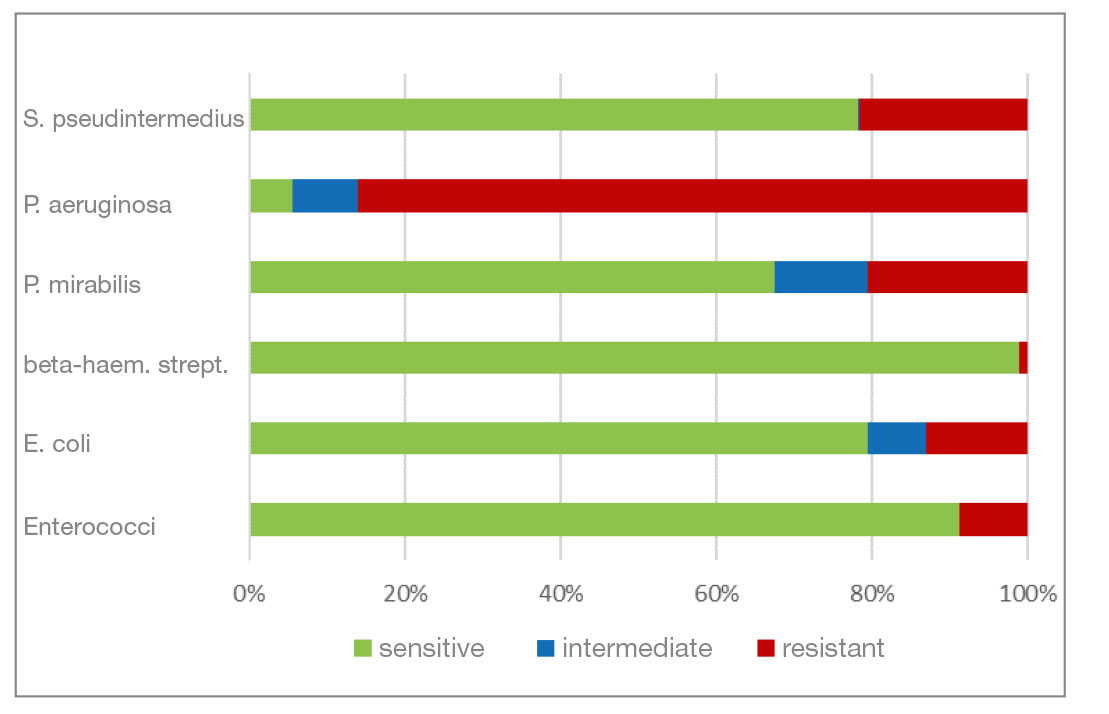
- Marbofloxacin and orbifloxacin: Both antibiotics belong to the group of fluoroquinolones (gyrase inhibitors). There was a higher percentage of P. aeruginosa isolates that were resistant to marbofloxacin. Resistance to orbifloxacin was increased in P. aeruginosa, P. mirabilis and E. coli. Overall, a favourable level of resistance could still be seen for both agents.
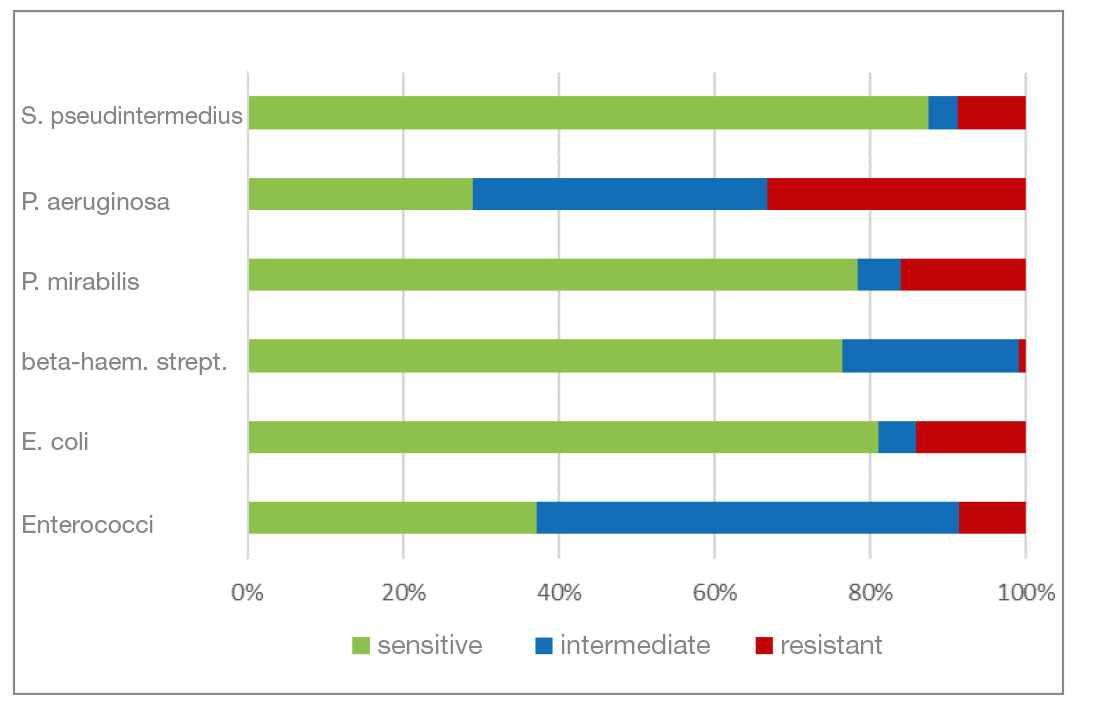
- Gentamicin and neomycin are aminoglycoside antibiotics. Both agents have a very similar resistance pattern. The level of resistance to gram-negative bacteria is good. Beta-haemolytic streptococci and enterococci show a natural resistance to aminoglycosides.
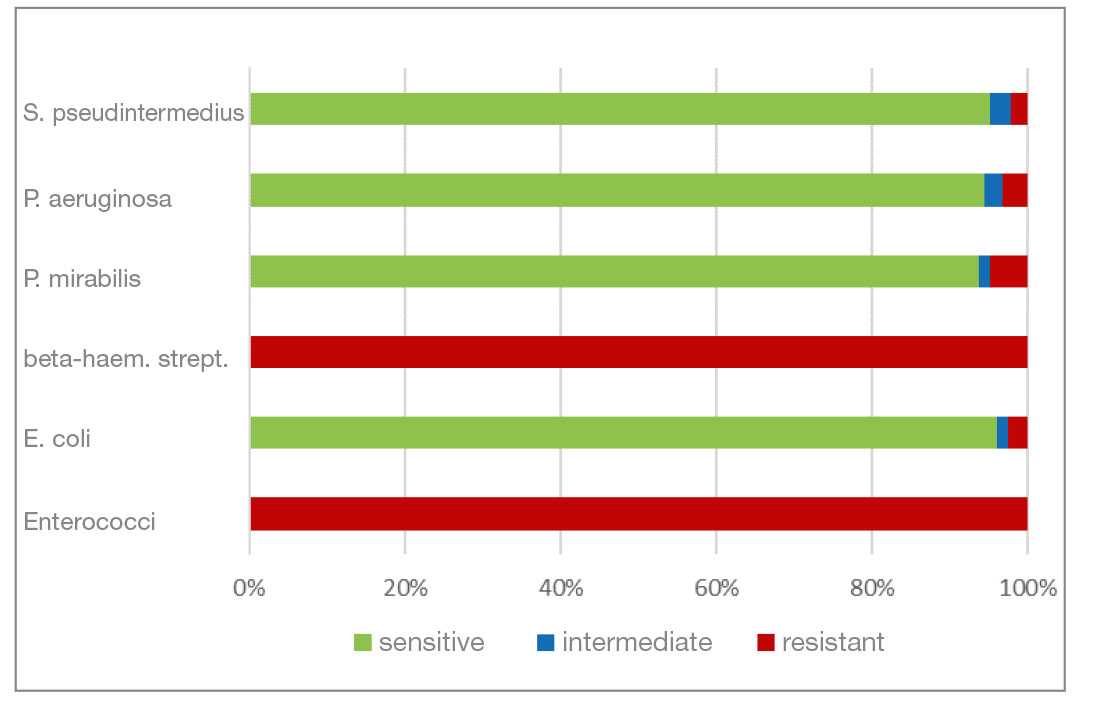
- Chloramphenicol and florfenicol belong to the fenicol group of antibiotics. They have a very broad spectrum of activity. In addition to P. aeruginosa isolates, S. pseudintermedius and P. mirabilis also show a higher percentage of resistant isolates here.
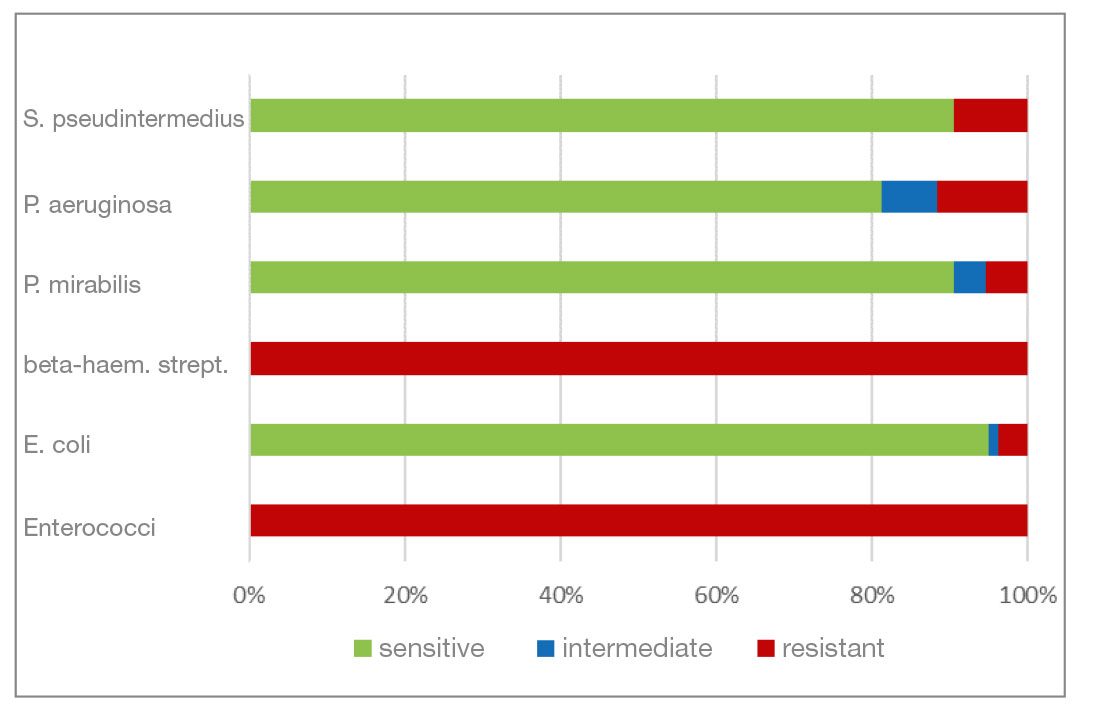
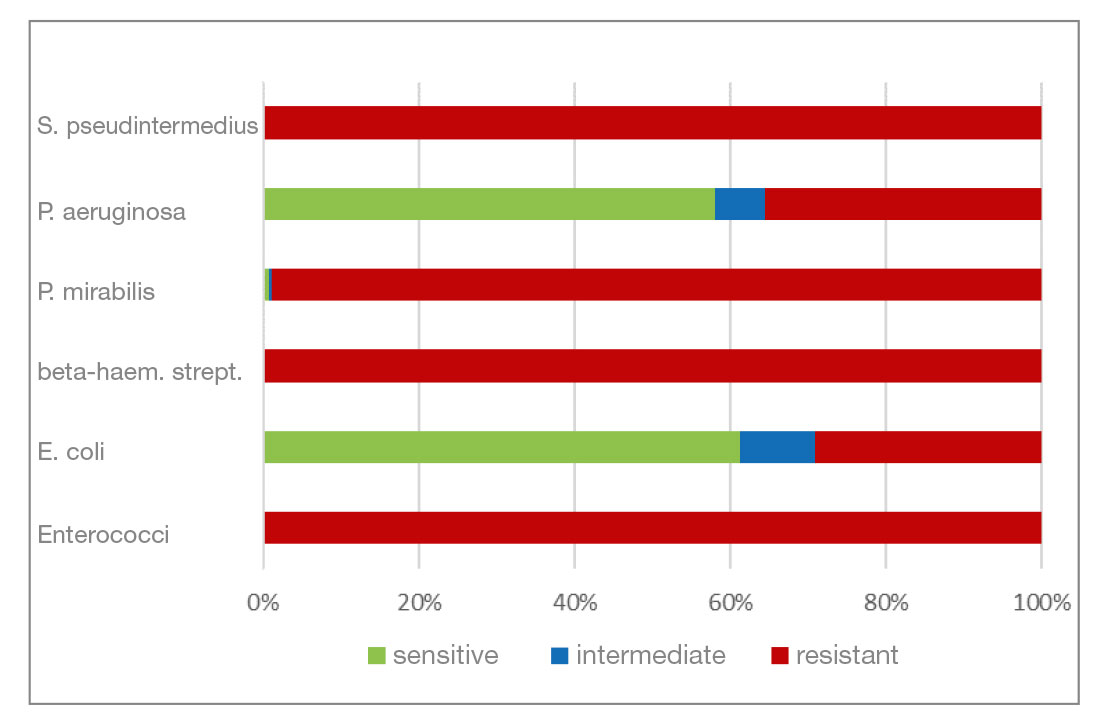
- Polymyxin B (polypeptide antibiotic) is effective against many gram-negative bacteria. Gram-positive bacteria are mostly resistant. In this analysis, gram-negative pathogens have a high percentage of resistant isolates (30% and more). All gram-positive bacterial isolates are resistant.
Conclusion:
In the present analysis, S. pseudintermedius, beta-haemolytic streptococci and P. aeruginosa have been the most frequently detected bacteria in O. e. in dogs. They are followed by E. coli, P. mirabilis and enterococci. P. aeruginosa, E. coli and P. mirabilis show a higher percentage of isolates resistant to polymyxin B. Apart from that, isolates are mostly sensitive to the other active substances, so that the level of resistance can be considered quite positive. The results of this analysis are consistent with those of previous studies (national and international ones) with regard to the most frequently detected bacteria and their resistance to the active substances contained in ear preparations, as well as in terms of the high detection rate of M. pachydermatis in mycologically positive samples. Otitis externa in dogs is a multifactorial disease that is not caused by bacteria or yeasts alone, but occurs because of a primary disease which needs to be identified for successful treatment. The analysis from 2016 took place before the new regulations of the German Veterinary Pharmacy Act (TÄHAV) came into force. According to the TÄHAV, it is now necessary to prepare an antibiogram when using fluoroquinolones (3rd/4th generation cephalosporins) which requires identification of the pathogen. From the laboratory’s point of view, it is generally recommended that practitioners have a culture examination carried out if they suspect a bacterial infection. An analysis of the current data in order to assess the development is in progress.
Dr. Corinna Hader
The laboratory can assist you with:
- cultivation and identification of pathogens
- detection of multi-resistant pathogens
- preparation of antibiograms to help determine which agent could be used
- detecting/excluding a mycological origin
- Fig. 1: Number of isolated pathogens per ear swab
- Fig. 2: Distribution of the most frequently detected bacteria in per cent
-
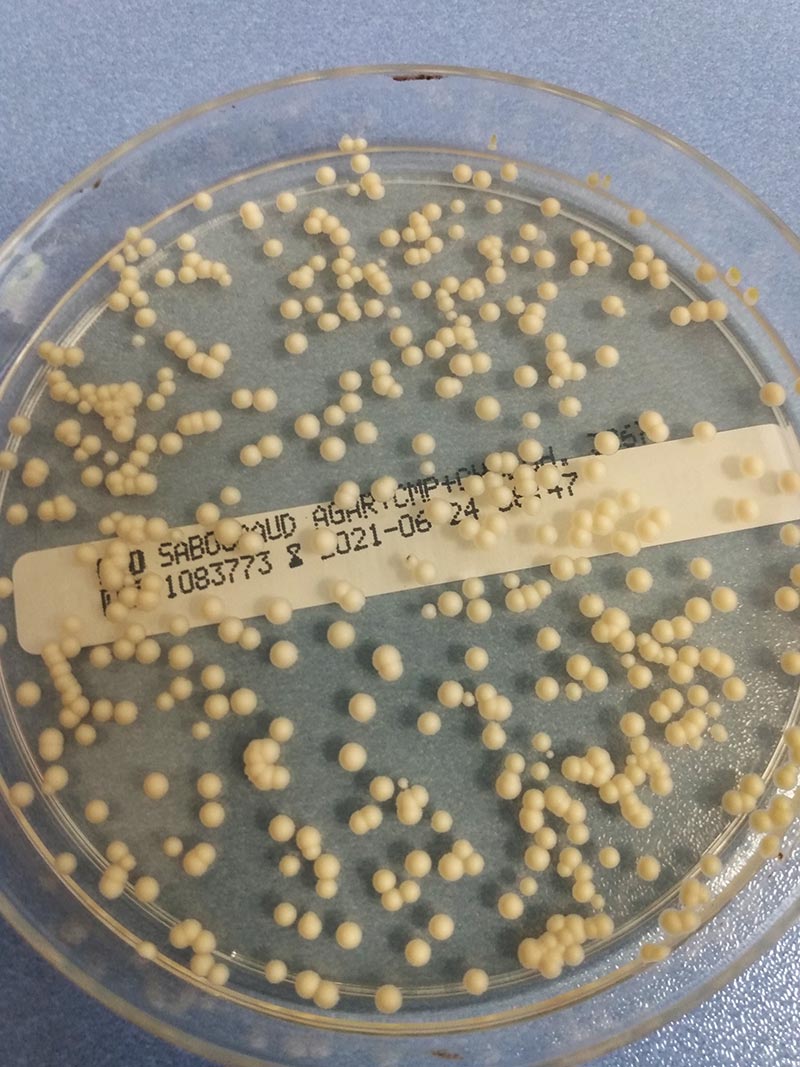
Figure 3: Malassezia colonies on Sabouraud chloramphenicol cycloheximide agar
photo credits: Laboklin
- Fig. 4: Findings of the mycological examination in combination with each of the detected bacterial isolates
- Fig. 5: Percentage of the most frequently detected bacteria classified as sensitive/intermediate/resistant to marbofloxacin
- Fig. 6: Percentage of the most frequently detected bacteria classified as sensitive/intermediate/resistant to orbifloxacin
- Fig. 7: Percentage of the most frequently detected bacteria classified as sensitive/intermediate/resistant to gentamicin
- Fig. 8: Percentage of the most frequently detected bacteria classified as sensitive/intermediate/resistant to neomycin
- Fig. 9: Percentage of the most frequently detected bacteria classified as sensitive/intermediate/resistant to chloramphenicol (florfenicol)
- Fig. 10: Percentage of the most frequently detected bacteria classified as sensitive/intermediate/resistant to polymyxin B