Physiology and tasks of the intestinal microbiota
The gastrointestinal microbiota is a complex community of microorganisms that colonise the digestive tract and play an important role in animal health and well-being. It consists of 99% anaerobic bacterial species that grow in the absence of atmospheric oxygen and belong, for example, to the bacterial strains Firmicutes, Proteobacteria and Fusobacteriota. However, the exact composition of the gut microbiota varies from individual to individual, changes throughout life and is strongly influenced by factors such as diet, diseases, medications and environmental conditions. A healthy gut microbiota supports digestion, immune defense, production of vitamins and short-chain fatty acids (SCFAs) and defense against pathogenic germs. To this end, the individual bacterial strains are closely interrelated, interact with each other in a process known as cross-feeding forming their own highly interconnected ecosystem.
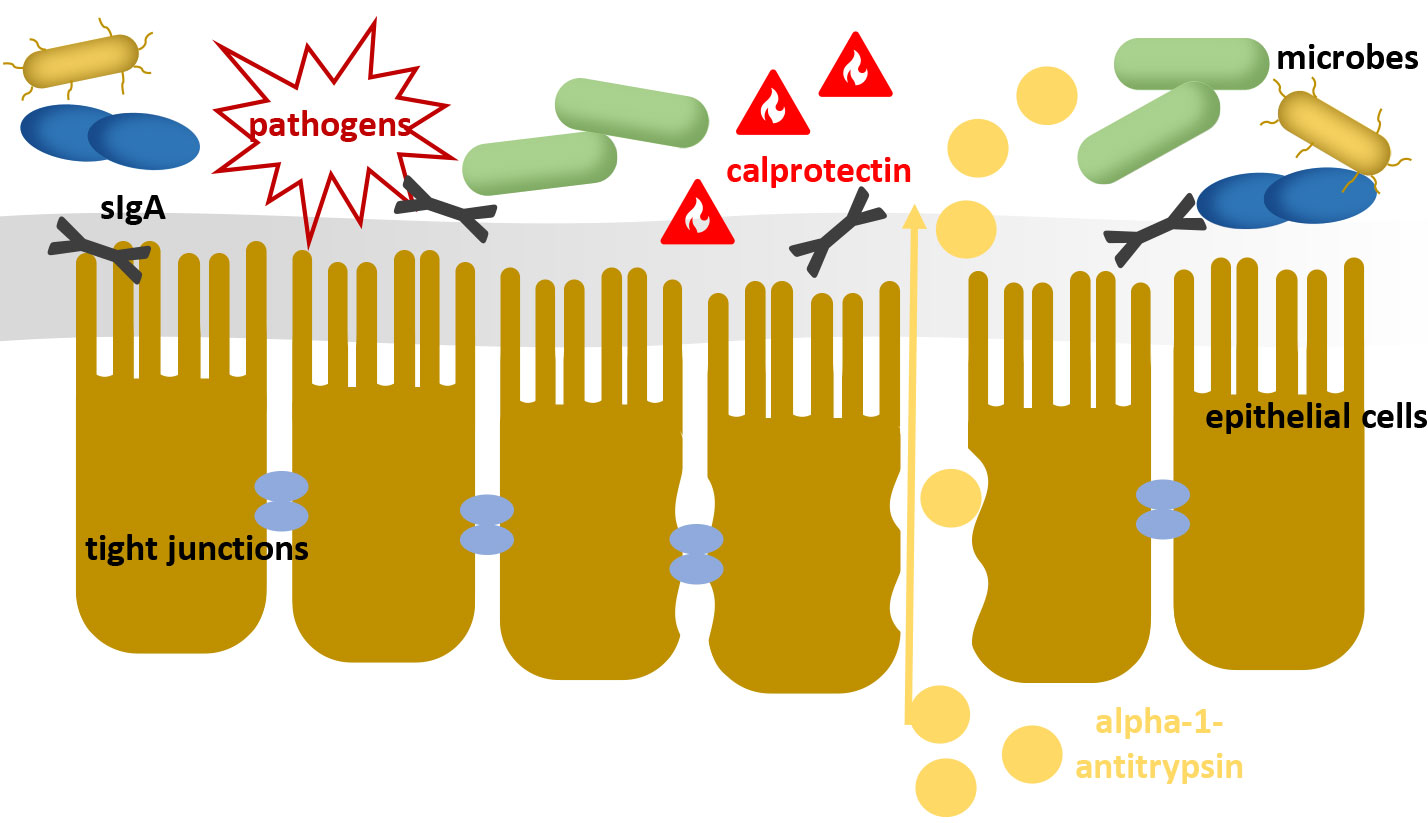
This ecosystem of the intestinal microbiota can be seen as the first level of the intestinal barrier. By its mere presence and metabolic performance, it prevents the colonisation of pathogens and impairs their proliferation (colonisation resistance). The subsequent mucus layer and the intestinal mucosa continue to provide a mechanical barrier against foreign germs and antigens. Intestinal epithelial cells are connected to each other by « tight junctions » (cell-cell connections), so that the transfer of substances occurs very selectively where the mucosa is intact. As the third level of the intestinal barrier, the gut-associated lymphoid tissue (GALT) plays an important role. The simple existence of the intestinal microbiota contributes to the maintenance of a defensive immunological barrier against foreign germs via constant training of the GALT. In addition, pathogens are actively repressed by stimulating the synthesis of antimicrobially active peptides such as ß-defensins and immunoglobulins. Decreased barrier function, e.g., due to bacterial imbalances, can lead, among other things, to the transfer of antigens, endotoxins centrally active metabolites from the intestinal lumen into the bloodstream which initiates or enhances diverse pathomechanisms.
Consequences and diagnostics of dysbiosis
Dysbiosis of the microbiota is a disturbance in the balance between different bacterial species that can lead to decreased diversity, increased numbers of potentially harmful bacteria and altered microbiota function. It can be triggered or facilitated by various factors, such as stress, dietary changes, antibiotic therapy, infections or chronic intestinal disease. Furthermore, in the presence of existing gastrointestinal symptoms, dysbiosis can lead to a rapid worsening of clinical symptoms. An analysis of dysbiosis e.g. by quantitative PCR (sample material: faeces) can therefore be a useful diagnostic tool to assess the extent of intestinal dysfunction and to enable targeted therapy.
Clinical symptoms that warrant dysbiosis analysis include but is not limited to:
- Chronic and acute inflammatory bowel diseases
- Flatulence, diarrhea, constipation
- Small intestinal overgrowth syndrome (SIBO), irritable bowel syndrome
- Maldigestion, malabsorption, micronutrient deficiencies
- Feed intolerances, allergies
- Itching, eczema
- Coat loss
-
Fig. 1: A damaged intestinal barrier loses its protective function against pathogens and pollutants
Image source: Laboklin
-
Fig. 2: Female dog Leila (left) and deposited feces (right)
Image source: Dr Jennifer Scherzer
-
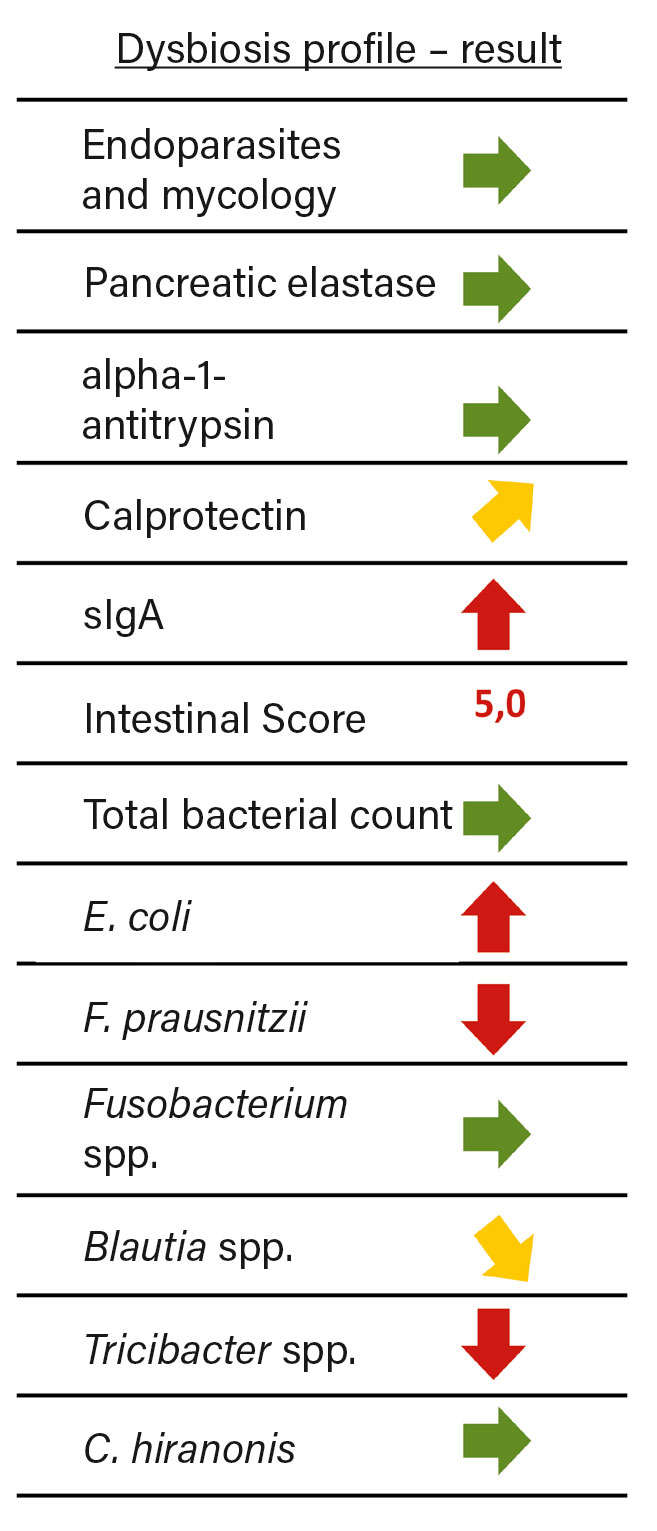
Fig. 3: Result of the dysbiosis profile of female dog « Leila ». Green arrows correspond to the reference values or show no abnormality, compared to the reference values yellow arrows indicate a slight increase or decrease and red arrows indicate a strong deviation.
Image source: Laboklin
Since disease patterns and clinical symptoms cannot necessarily be traced back to a single bacterial strain or species, diagnostics focus on the measurement of entire functional bacterial groups. From studies, some bacteria and bacterial groups have been identified in dogs and cats that can be used as marker bacteria for a dysbiotic state of the intestine. The detection of the marker bacteria by molecular biological methods can provide information about the colonisation resistance. On the other hand, the observation of individual functional bacterial groups, such as SCFA-forming or mucosa-protective bacteria, can allow conclusions to be drawn about the condition of the mucus layer and the energy supply of the intestinal epithelial cells.
However, it should be noted that the causes of dysbiosis can be very diverse and the disturbance of the bacterial balance has a direct influence on the barrier function of the intestinal wall. Thus, the determination of further parameters can be helpful to look at the condition of the intestinal barrier in more detail (Figure 1).
Faecal biomarkers for the assessment of intestinal mucosa and digestive performance
In addition to the marker bacteria, the following biochemical parameters may supplement the dysbiosis profile:
- Calprotectin – is well suited as an inflammatory marker in differential diagnostics for a number of diseases of the gastrointestinal tract (e.g. IBD or other inflammatory bowel diseases). Likewise, it can be used to monitor the course of therapy and detect recurrences in these diseases.
- Alpha-1-antitrypsin – can be used as a marker of protein loss through the intestine. However, it should be noted that this is not a marker specific for inflammatory bowel disease. Elevated levels occur in other gastrointestinal or systemic diseases that result in increased intestinal permeability (« leaky gut »).
- The concentration of canine pancreatic elastase 1 – directly reflects exocrine pancreatic A deficiency in pancreatic elastase can lead to digestive problems and malnutrition. A low value in the faeces indicates a lack of digestive enzyme production. Decreased precaecal digestive efficiency also leads to increased flooding of fats and proteins into the colon. This shifts the milieu in favor of potentially detrimental germs and may favor the development of dysbiosis.
Another biomarker, secretory IgA (sIgA), can provide information about the burden on the intestinal mucosa and will be newly offered in the dysbiosis profile for dogs and cats from 01.07.2023.
Significance and function of the sIgA
The antibody sIgA, which is found primarily on mucous membranes, serves as a first line of defense against pathogens that can enter through the respiratory tract, gastrointestinal tract or skin. As an important component of the GALT, it binds to potentially harmful microbes or antigens, preventing them from docking or damaging the intestinal wall.
The relationship between the gut microbiome and sIgA is not yet fully clarified, but the microbiota has an impact on the GALT and thus on the formation and function of sIgA. On the one hand, it promotes the maturation and activation of plasma cells that produce sIgA. On the other hand, sIgA modulates the composition and activity of the intestinal microbiota by selectively binding or inhibiting certain microbes. This creates a symbiotic relationship that is important for the maintenance of the intestinal barrier and mucosal immunity.
sIgA is an important diagnostic marker for various diseases of the gastrointestinal tract, such as chronic inflammatory enteropathy or leaky gut syndrome. The determination of sIgA in the faeces can provide information about the functional capacity and the of the intestineassociated immune system.
- Decreased detection of sIgA may indicate impairment of the intestinal barrier and weakening of the local immune system.
This may be associated with increased susceptibility to intestinal disease, increased susceptibility to infection, allergic reactions, or immunosuppressive conditions. - Elevated sIgA may indicate a particular strain on the intestinal immune system, which may be caused by acute or chronic inflammation of the intestinal mucosa. Possible conditions include colitis, IBD or parasitosis.
The determination of sIgA in faeces is a simple and non-invasive method to assess the local immune status in the intestine. However, it should always be interpreted in combination with other clinical and laboratory parameters to make a correct diagnosis.
Case study
« Leila » is an 8-year-old female boxer who has been suffering from recurrent diarrhea (Figure 2), flatulence and loss of appetite for several months.
A dysbiosis profile was obtained from collected faeces (Figure 3). The analysis revealed elevated levels of sIgA and calprotectin. This indicates inflammation of the intestinal mucosa as well as a strong stress of the local immune system. Analysis of marker germs revealed decreased diversity of the microbiome. The intestinal score of 5.0 was in the abnormal range, indicating a dysbiotic state. A closer look at the marker bacteria groups revealed a slight to strong reduction to the reference values of F. prausnitzii, Turicibacter spp. and Blautia spp. These species belong to the Firmicutes phylum and play a crucial role in the energy supply of intestinal septate cells as SCFA producers or SCFA recyclers.
Proteobacterium E. coli, on the other hand, was elevated compared to the reference range. This is observed in dogs in connection with gastrointestinal disorders. In addition to displacement of mucosa-protective bacteria, the strong immunogenic effect and adverse metabolites may contribute to irritation of the intestinal mucosa. Furthermore, the intestinal milieu is thus shifted in favor of pathogens.
By switching to an easily digestible feed with the addition of prebiotics, the growth of mucosal protective bacteria can be promoted while inhibiting the spread of detrimental bacteria. This effect can be further enhanced by the additional use of probiotics. Consequently, the enterocytes are again better supplied with energy and the consequences of irritation by harmful bacterial metabolites decrease.
If necessary, the therapy can be supplemented by the addition of anti-inflammatory drugs.
Dr Jennifer Scherzer